Biological Approach to Revitalization in Immature Permanent Teeth: No-instrumentation Technique
07/01/2026
Fellow
Warning: Undefined variable $post in /home/styleendo/htdocs/styleitaliano-endodontics.org/wp-content/plugins/oxygen/component-framework/components/classes/code-block.class.php(133) : eval()'d code on line 2
Warning: Attempt to read property "ID" on null in /home/styleendo/htdocs/styleitaliano-endodontics.org/wp-content/plugins/oxygen/component-framework/components/classes/code-block.class.php(133) : eval()'d code on line 2
Treatment for immature, necrotic permanent teeth is showing a paradigm shift in moving from a non-biological, barrier-creating approach (apexification) to a biological, tissue-regeneration approach. Conventional root canal treatment has a high long-term success rate (over 86%) for mature permanent teeth with irreversible pulpitis, pulp necrosis, or pulp exposure.
However, immature permanent teeth present more significant challenges and have a less predictable outcome due to their fragile root anatomy and physiology. Regenerative endodontics has emerged as a compelling alternative, provided that the case and patient meet the necessary criteria. For immature permanent teeth with pulp necrosis, regenerative endodontic procedures (REPs) offering a tissue-engineering approach aimed at revitalizing the pulp-dentin complex, are growing in popularity. This biological treatment promotes continued root development (due to the still present Hertwig’s Epithelial Root Sheath; HERS and, Stem Cells of Apical Papilla; SCAP), thereby increasing the tooth's long-term survival and restoring its physiological function. The objective of this case report is to present a successful regenerative procedure, detailing the clinical and radiographic findings from a 30-month observation period.
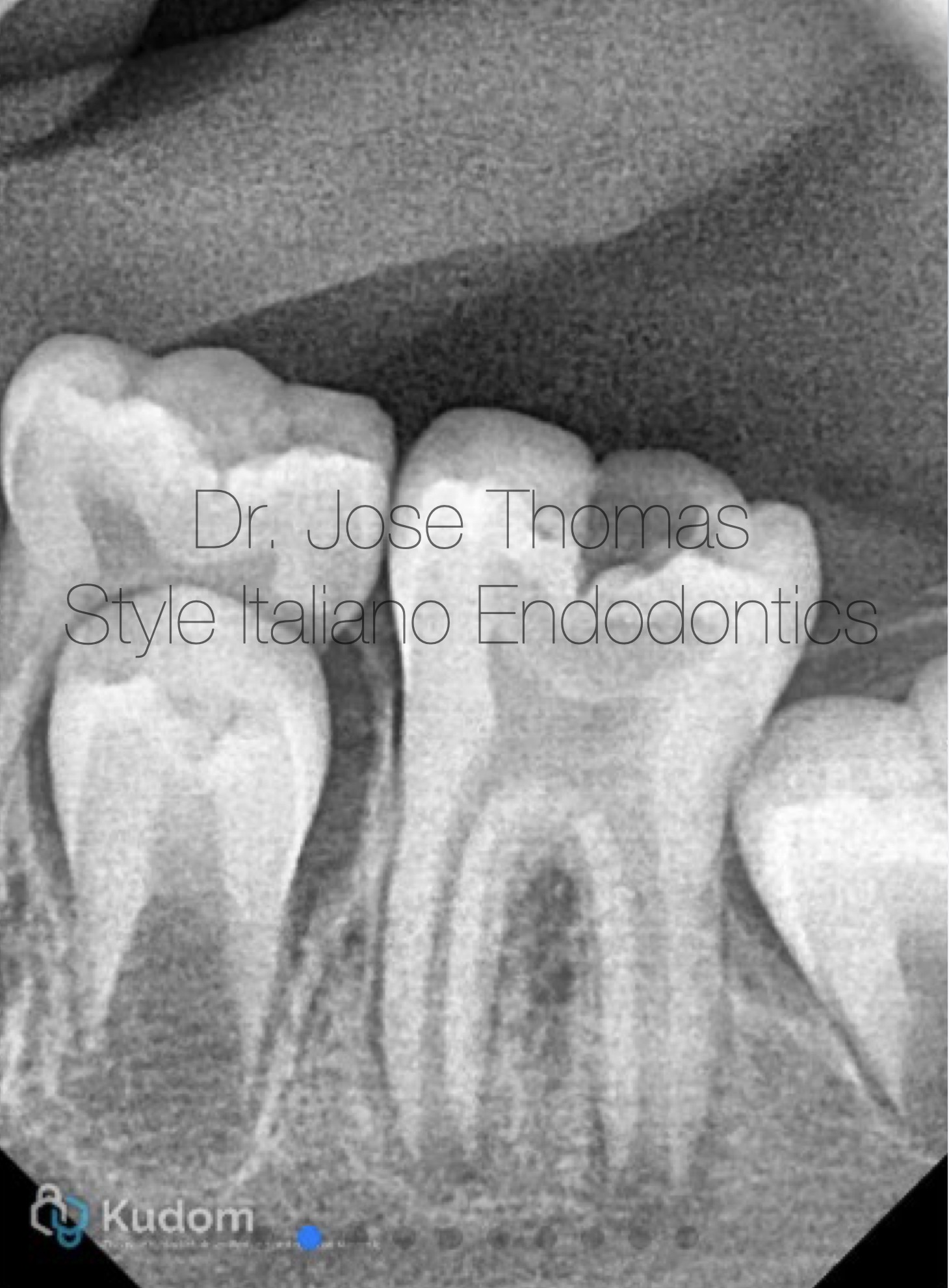
Fig. 1
The first visit was dedicated to disinfection of the root canal system. Treatment was started after rubber dam isolation and access was obtained. After the canal orifices were located they were copiously irrigated with saline to flush out loose necrotic debris. Mechanical instrumentation was avoided to retain the dentinal thickness around canals. Paper points were used to obtain tactile feedback of resistance, which would be from the presence of gel like necrotic tissue in the canal.
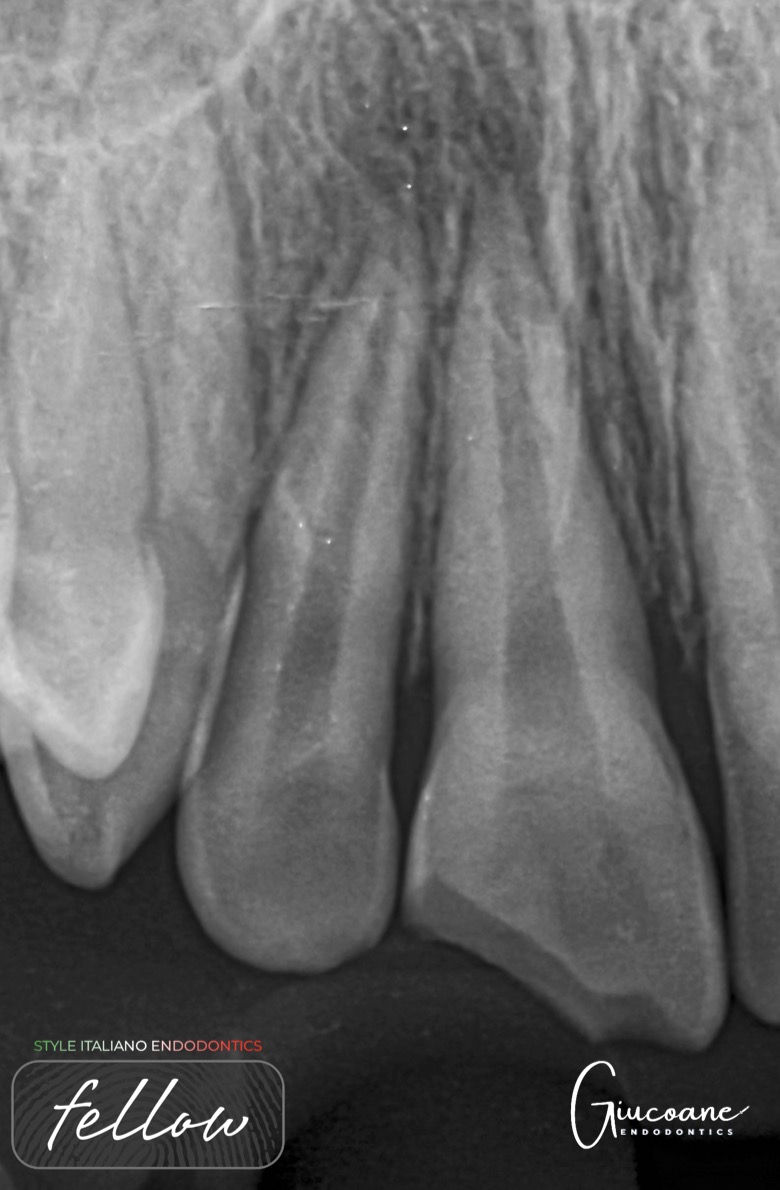
Xray Shows deep carious lesion in tooth 36. The root length is nearly complete with wide open apical foramina and indistinct apical lamina dura around both mesial and distal roots.
The length at which tactile feedback was received was considered to be the working length. The canals were then irrigated with 20 mL of 1% sodium hypochlorite for 5 minutes. A side-vented needle was used to deliver the irrigant, with the tip placed 2 mm short of the determined working length.
Following irrigation with sodium hypochlorite, the canal was thoroughly flushed with 5 mL of sterile physiological saline to neutralize residual irrigant in the canals and protect periapical tissues. The canals were then dried using sterile paper points.
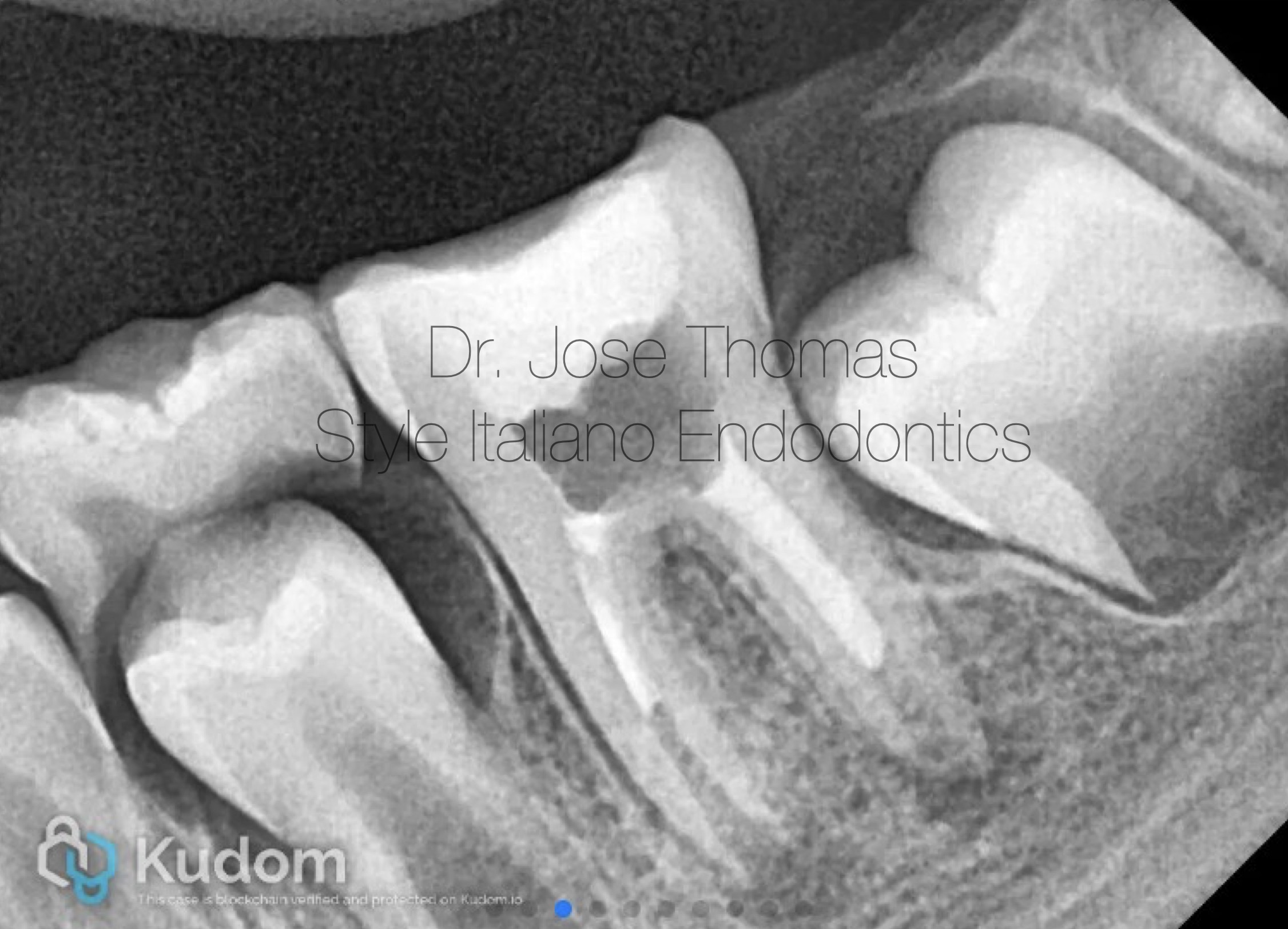
Fig. 2
A final irrigation was done with 20 mL of 17% EDTA to reverse the toxic effects of sodium hypochlorite, and to recover the cell viability. The pulp chamber and canals were dried with sterile paper points and calcium hydroxide medicament (Ultracal XS; Ultradent Products Inc., South Jordan, Utah) was placed loosely into the canal orifices without pressure, cavity closed with glass ionomer cement and patient was recalled after 3 weeks.
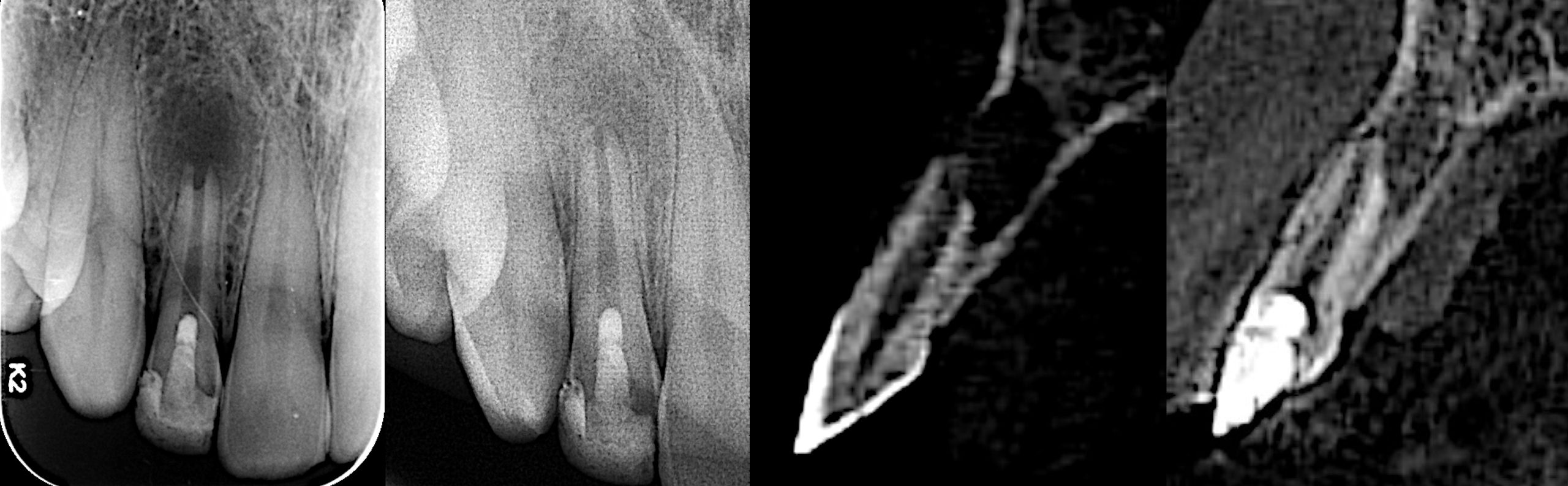
Xray shows the placement of calcium hydroxide medicament in the canals.
At the end of 3 weeks the patient reported with resolution of all symptoms. Canals were re-accessed and calcium hydroxide medicament was removed with copious sterile saline irrigation. The canals were then irrigated for 5 minutes with 20 mL of 17% EDTA. A side-vented needle was used for delivery, maintaining the tip 2 mm short of the established working length. Final irrigation with EDTA is shown to release growth factors on the radicular dentine surface which promotes cell recruitment, proliferation and differentiation and might therefore be beneficial for regenerative procedures.
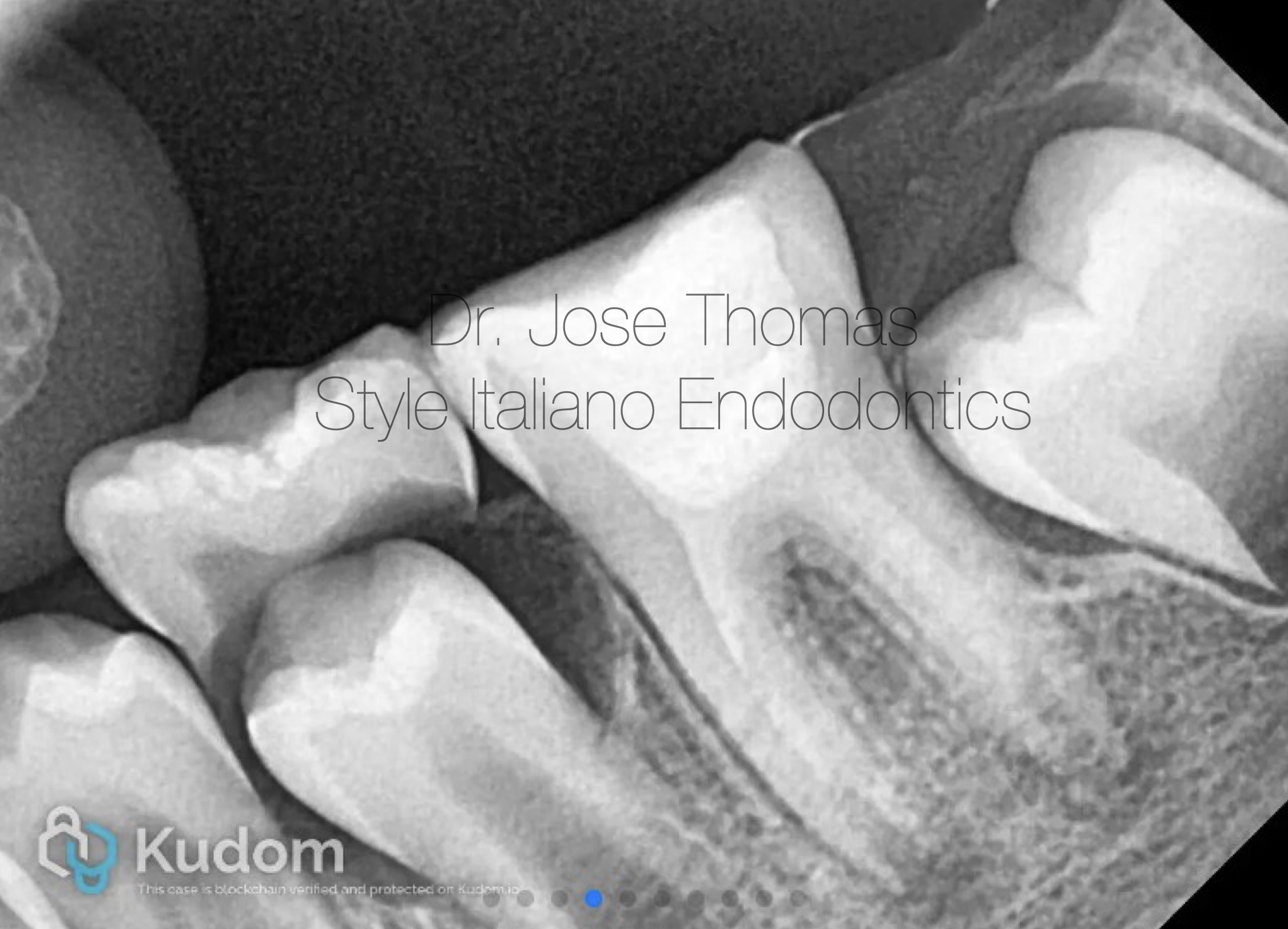
Fig. 3
The canals and chamber were then thoroughly dried with paper points and Biodentine® (Septodont, Saint-Maur-des-Fossés, France) was placed into the canals and up to the working length in small increments and gently condensed with a hand plugger. A resin modified GIC liner was used to close off the canal orifices, followed by restoration of coronal seal with composite build-up. Radiograph of 36 showing Biodentine® in canals and access closed with composite.

Fig. 4
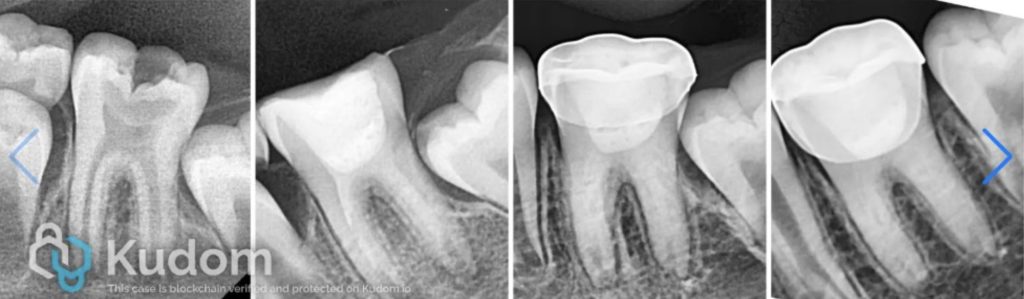
The patient received a stainless-steel crown at a follow-up appointment one week later. Periodic follow-up was done till 30 months.
Xray after cementation of stainless steel crown
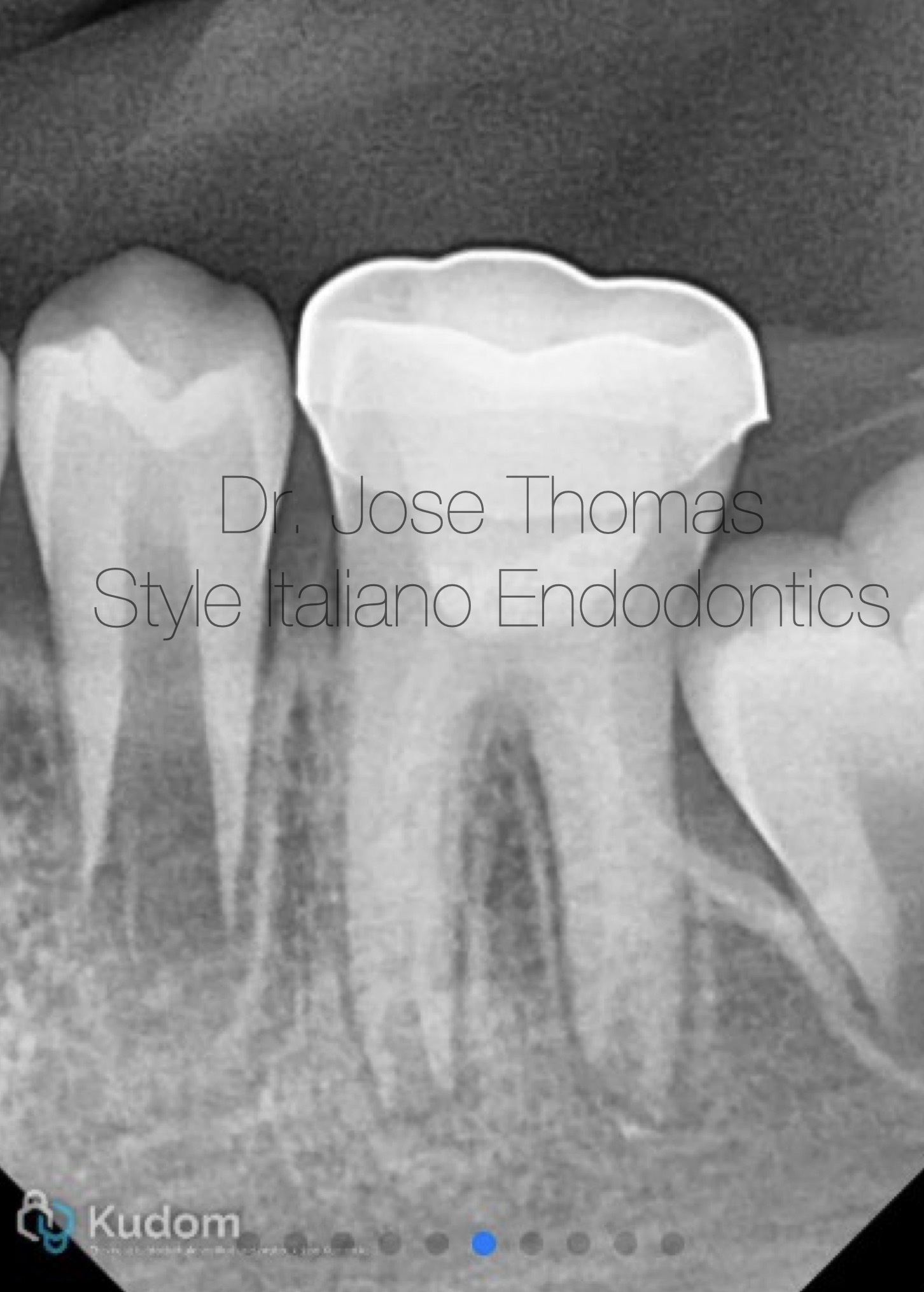
Fig. 5
Follow-up IOPA at 6 months
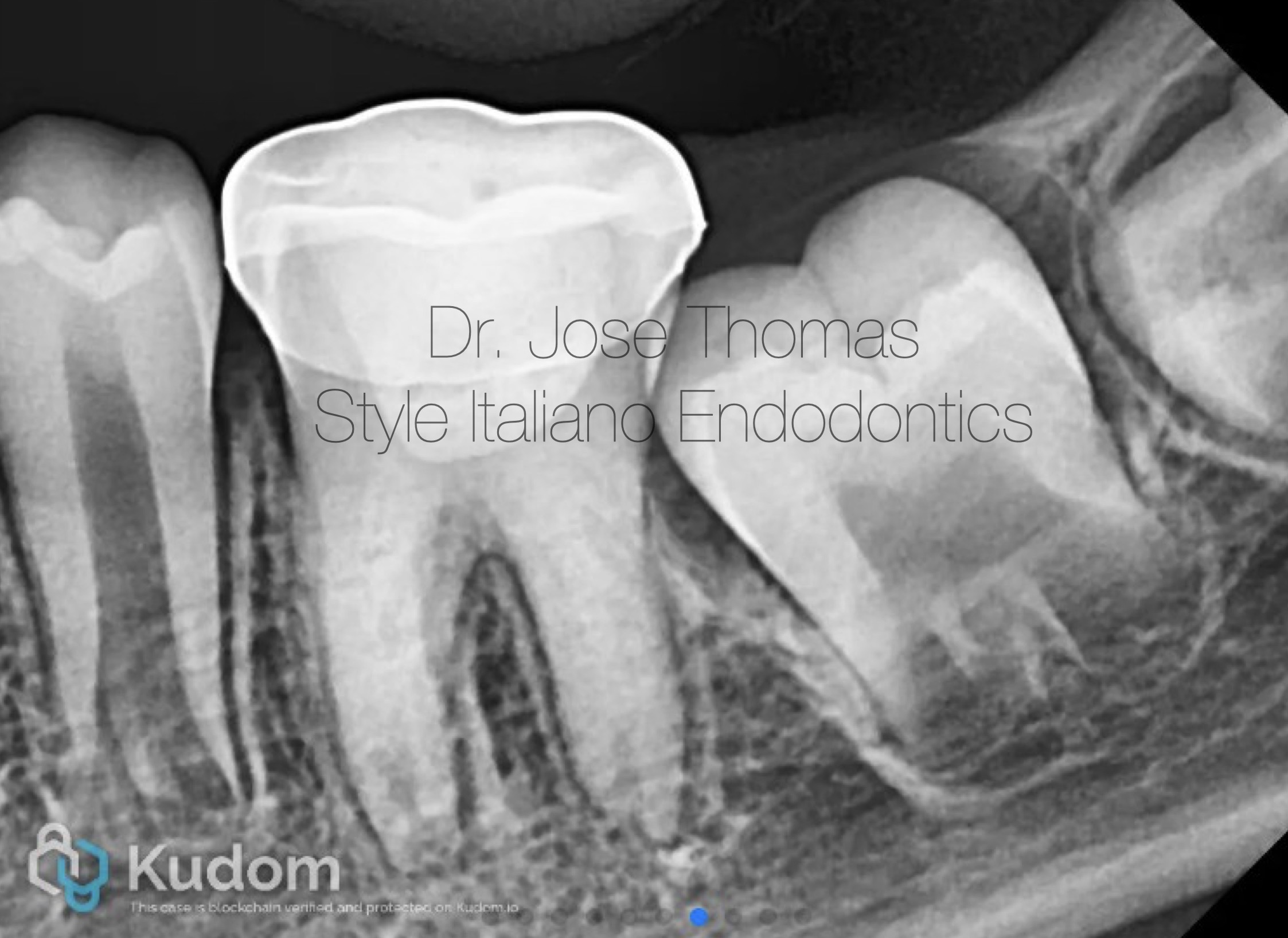
Fig. 6
Follow-up IOPA at 12 months
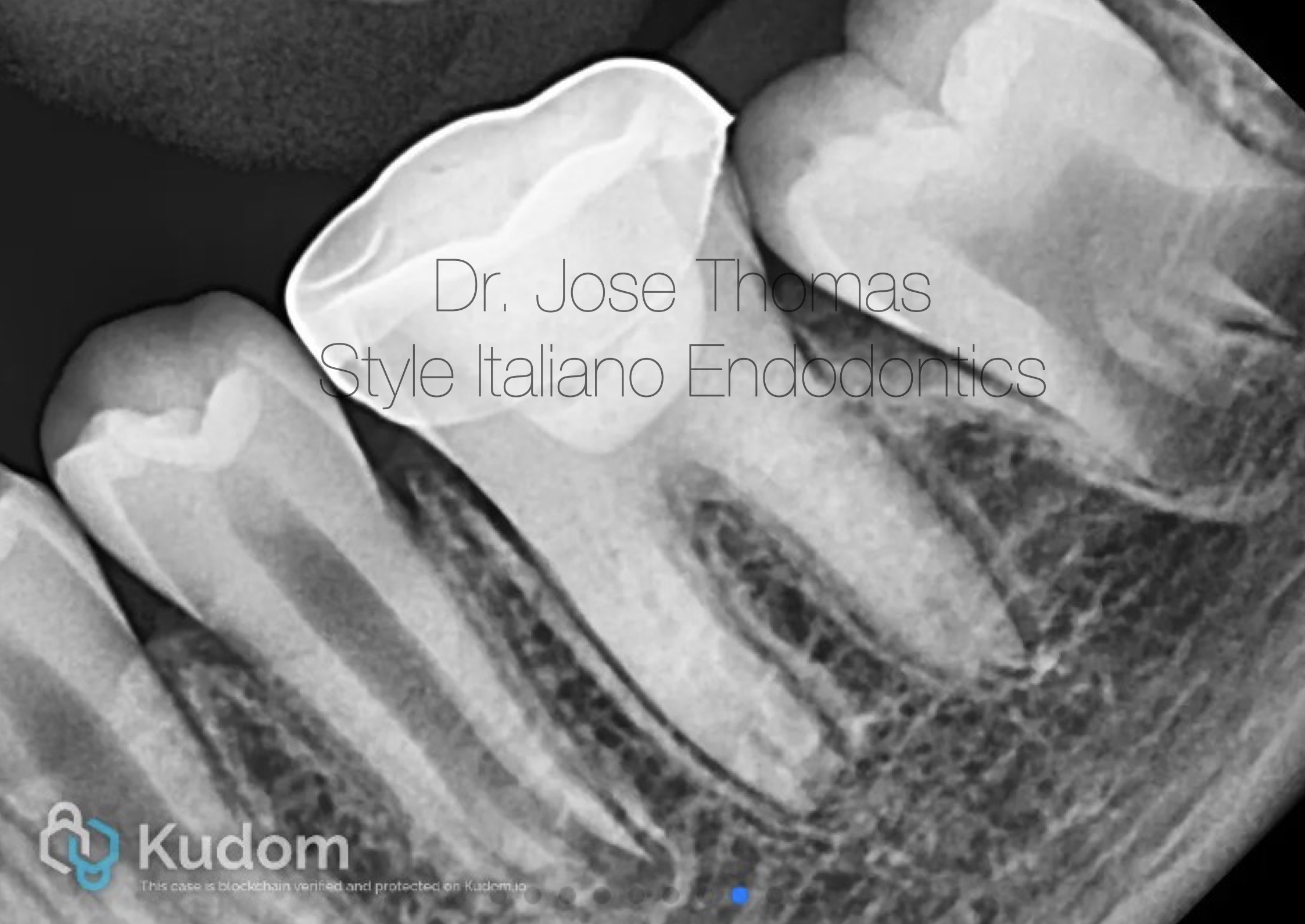
Fig. 7
Follow-up IOPA at 18 months
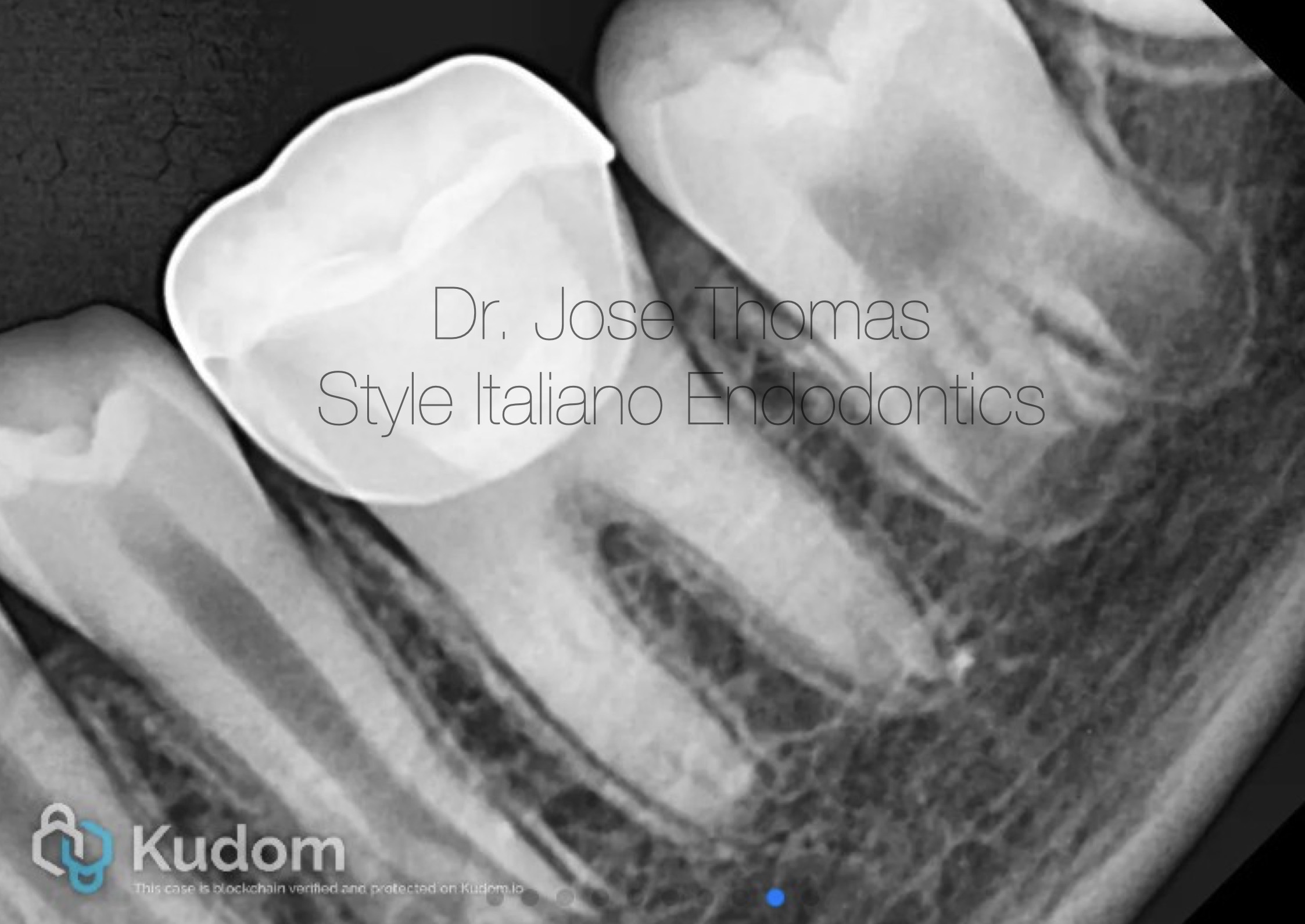
Fig. 8
Follow-up IOPA at 24 months
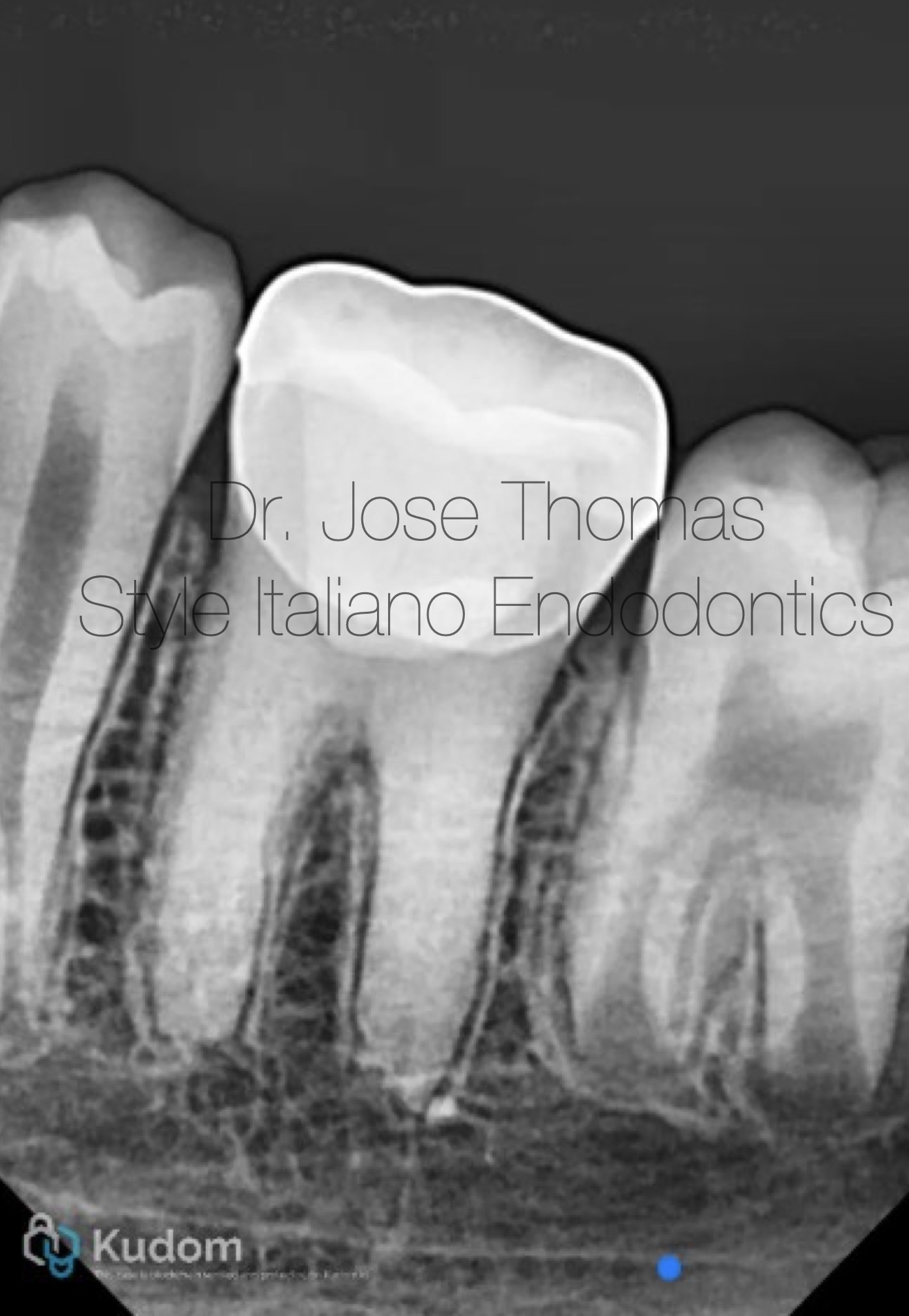
Fig. 9
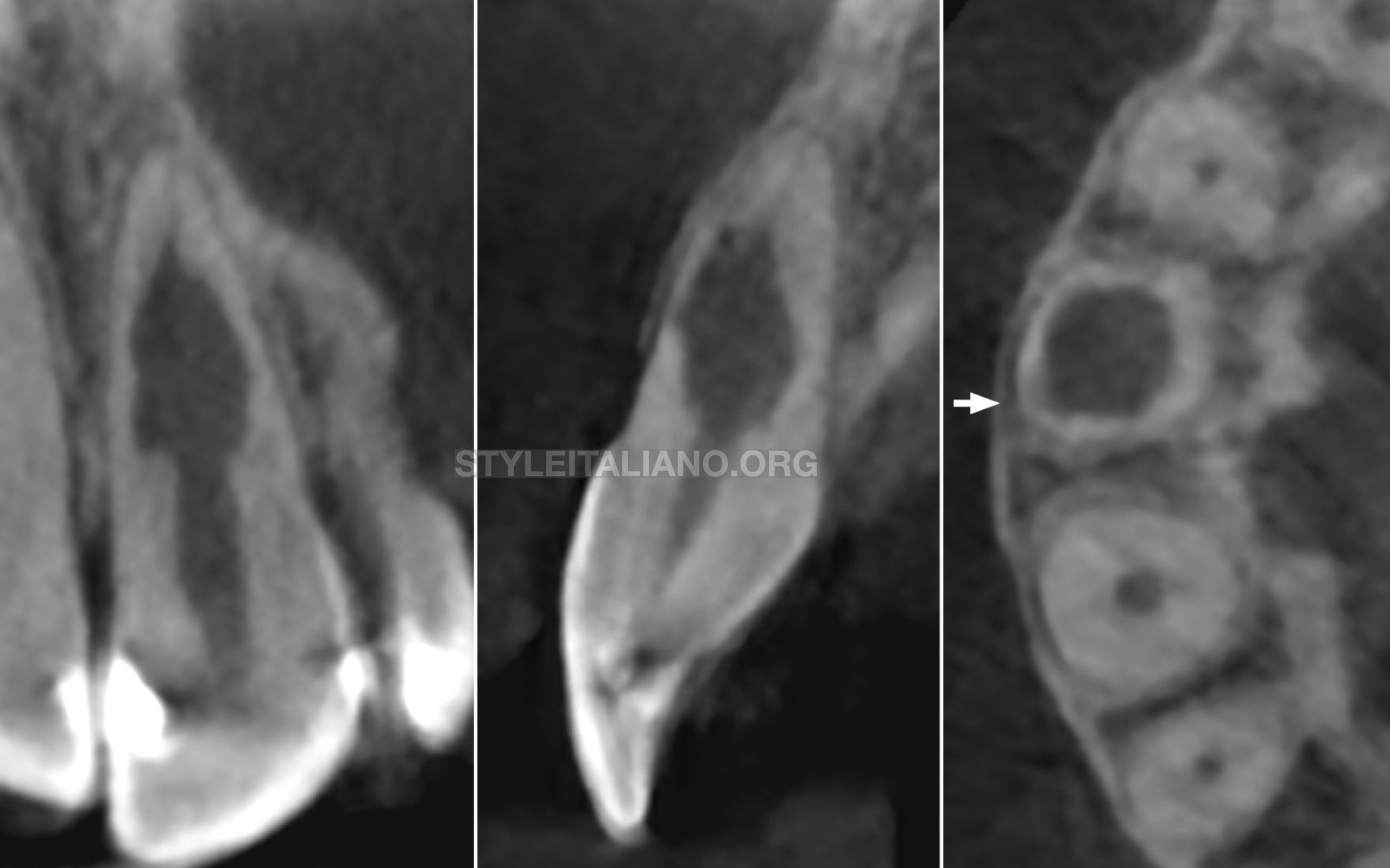
Follow-up radiograph at 30 months showed resolution of periapical lesion, and completion of root formation and establishment of lamina dura.
Xray IOPA at 30 months review shows resolution of periapical lesion, and completion of root formation and establishment of lamina dura

Fig. 10
Conclusions
This report demonstrates the successful management of an immature symptomatic permanent mandibular molar through regenerative endodontic procedure using tricalcium silicate-based material Biodentine®. This facilitated a predictable and minimally invasive restoration of the tooth by means of radicular regeneration.