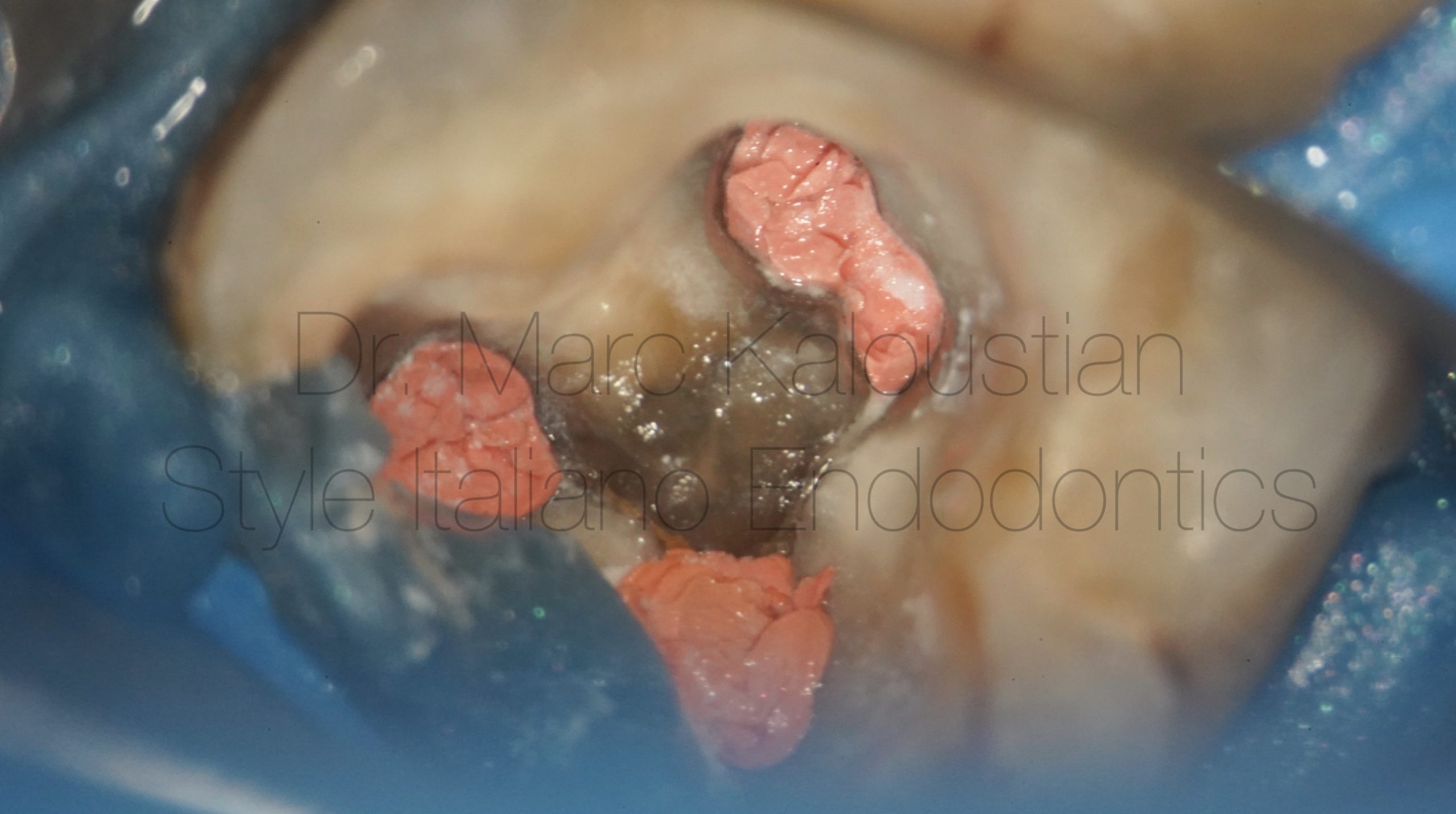
Apical lesion from formation to healing
29/07/2023
Fellow
Warning: Undefined variable $post in /home/styleendo/htdocs/styleitaliano-endodontics.org/wp-content/plugins/oxygen/component-framework/components/classes/code-block.class.php(133) : eval()'d code on line 2
Warning: Attempt to read property "ID" on null in /home/styleendo/htdocs/styleitaliano-endodontics.org/wp-content/plugins/oxygen/component-framework/components/classes/code-block.class.php(133) : eval()'d code on line 2
Apical periodontitis serves an essential protective function aimed at confining bacteria discharged from the root canal space and preventing them from spreading into adjacent bone marrow spaces and other remote sites. The process is unique in the sense that it cannot eradicate the source of infection. The reason is that once a pulp has become necrotic, defence mechanisms cannot operate far into the root canal owing to the lack of vascular support. Although these mechanisms can act at the apical margins of the necrotic tissue, they are unable to penetrate it in a fully developed tooth. Consequently, without proper endodontic treatment, apical periodontitis may prevail chronically. Bone resorption is a most conspicuous feature of apical periodontitis and is an unavoidable side-effect of the defensive process. Some may view it as a "price" paid by the host to provide the necessary, effective immune response to the root canal infection. On a microscopic level, different structural frameworks of apical periodontitis can be identified. These forms include apical granuloma, apical abscess, and apical cyst. Clinically and radiographically, these histopathological entities cannot be distinguished from each other or recognised, with the exception of abscesses with a sinus tract. Apical granuloma is the most common form of apical periodontitis and consists of an inflammatory lesion dominated by lymphocytes, macrophages, and plasma cells. Apical abscess denotes the presence of pus within the lesion. Abscess formation may reflect a shift in cellular dynamics within a pre-existing apical granuloma or be a direct outcome of an acute primary infection. An apical cyst is an epithelium-lined cavity that contains fluid or semi-solid material and is commonly surrounded by dense connective tissue variably infiltrated by mononuclear leukocytes and PMNs.Apical cysts are divided into pocket cysts (bay cysts) and true cysts. A pocket cyst is an apical inflammatory cyst that contains a cavity that is open to and continuous with the root canal space. True apical cysts, on the other hand, are located within the periapical granuloma with no apparent connection between their cavity and that of the root canal space.
Mechanism of formation of periapical lesion. Cells and functions in the periapical granuloma. Bacterial antigens derived from the infected root canal are taken by antigen-presenting cells (APC), processed, and presented to the T-lymphocytes. A dual signal of antigen presentation with IL-1 activates the T-lymphocytes. Cytokines produced by these activated cells include IL-4, IL-5, and IL-6, which induce proliferation and maturation of a specific clone of B-lymphocytes that were exposed to this specific antigen, to result in plasma cells producing IgG specific to this antigen; INFγ, which serves to activate macrophages which in turn will produce the IL-1 essential for local recruitment of circulating PMNs and IL-8 which activates these PMNs. Bacterial endotoxin (LPS), derived from Gram-negative bacteria, synergistically participates in the activation of macrophages. All of the above is aimed at allowing effective specific phagocytosis by the PMNs of any bacterium emerging from the apical foramen. Bone resorption is a side-effect of the above defensive process, mediated by TNFβ, produced by the activated T-lymphocytes, and IL-1β, produced by the activated macrophages. Both activate osteoclastic bone resorption. Bacterial elimination in periapical lesions is carried out by PMNs. All other constituents and processes in apical periodontitis may be viewed as serving this ultimate goal. That's why Neutropenic patients may have spontaneous necrosis and abscess formation in relation to mild caries. Bone resorption produced by activated macrophages, and TNFβ, produced by activated T-lymphocytes, are responsible for the local bone resorption in apical periodontitis. Nevertheless, osteoclasts do not have receptors for these cytokines and require other cells to trigger them. IL-1β and TNFβ engage receptors on osteoblasts and bone stromal cells, thus triggering these cells to express the surface ligand osteoprotegerin ligand (OPGL, RANK ligand. OPGL- receptors (receptor activator of nuclear factor κB, RANK) are found on preosteoclasts and on osteoclasts. Engagement of the receptors on nearby preosteoclasts activates these cells to become multi-nucleated osteoclasts. Similar engagement of the receptors on existing osteoclasts activates them to express an active ruffled border and resorb bone. Both OPGL (RANK ligand) and its receptor on the preosteoclasts and osteoclasts (RANK) are cell-bound molecules.
This activation process is down-regulated by a soluble mediator, osteoprotegerin (OPG), which competitively inhibits osteoclast activation by binding to the OPGL, thus blocking it and preventing the engagement of the receptors on the osteoclasts and preosteoclasts (51). This process explains how osteoclasts, which do not have IL-1β- or TNFβ-receptors, are locally activated by these cytokines. The apical granuloma may occasionally develop into an acute apical abscess or into a chronic abscess. The reason is a shift in the bacteria-host equilibrium. Healing The cytokines TNFβ and IL-1β, which are produced by activated T-lymphocytes and activated macrophages in the lesion, serve as the primary main signals that induce osteoclastic bone resorption in an apical granuloma. The surrounding bone is a rich source of osteoprogenitor cells that, together with locally produced EGF, IGF, TGFβ, PDGF, and BMPs, provide osteoblastic potential. Once bacteria are eliminated from the root canal, a gradual yet slow reduction of the local production of TNFβ and IL-1β occurs, and the osteogenic potential begins to dominate, causing healing of the lesion by new bone apposition. How long does it take the lesion to heal? Several large-scale follow-up studies indicate that 74–85% of apical lesions heal within 48 months. Of the lesions that eventually healed, only 50% were healed or in the process of healing at 6 months after treatment, namely 42.5% of the total number of lesions that were studied (50% of 85%). At 12 months, 88% of the lesions that eventually healed were healed or in the process of healing, namely 75% of the total number of lesions (88% of 85%)
What factors can have an influence on the healing of the apical process? Host-related factors such as neutropenia, diabetes mellitus, and smoking. Pharmacology-related factors such as estrogen, bisphosphonates, and metformin reduce bone resorption. Operator-related factors such as inefficient debridement, over-instrumentation, and over-obturation. An extraradicular infection (that does not respond to conventional endodontic treatment) and a foreign body granuloma. Extraradicular foreign materials have been reported as another cause of the persistence of apical lesions. Such foreign materials may include materials used in root canal treatment, such as minute contaminated particles of gutta-percha, cellulose particles originating from paper points, and the cotton wool that were extruded into the apical tissues, especially when associated with trauma to the apical tissue, and endodontic sealants and calcium salts derived from apically extruded Ca(OH)2. Another possible source of foreign material is food that is pushed into a root canal that is left open during treatment, as in this case, in which leguminous seeds (pulses) were found in an apical granuloma that did not respond to treatment. The presence of such foreign materials that are extruded during root canal treatment may keep the macrophages in the apical lesion in a perpetually activated state, thus preventing the healing of the lesion.
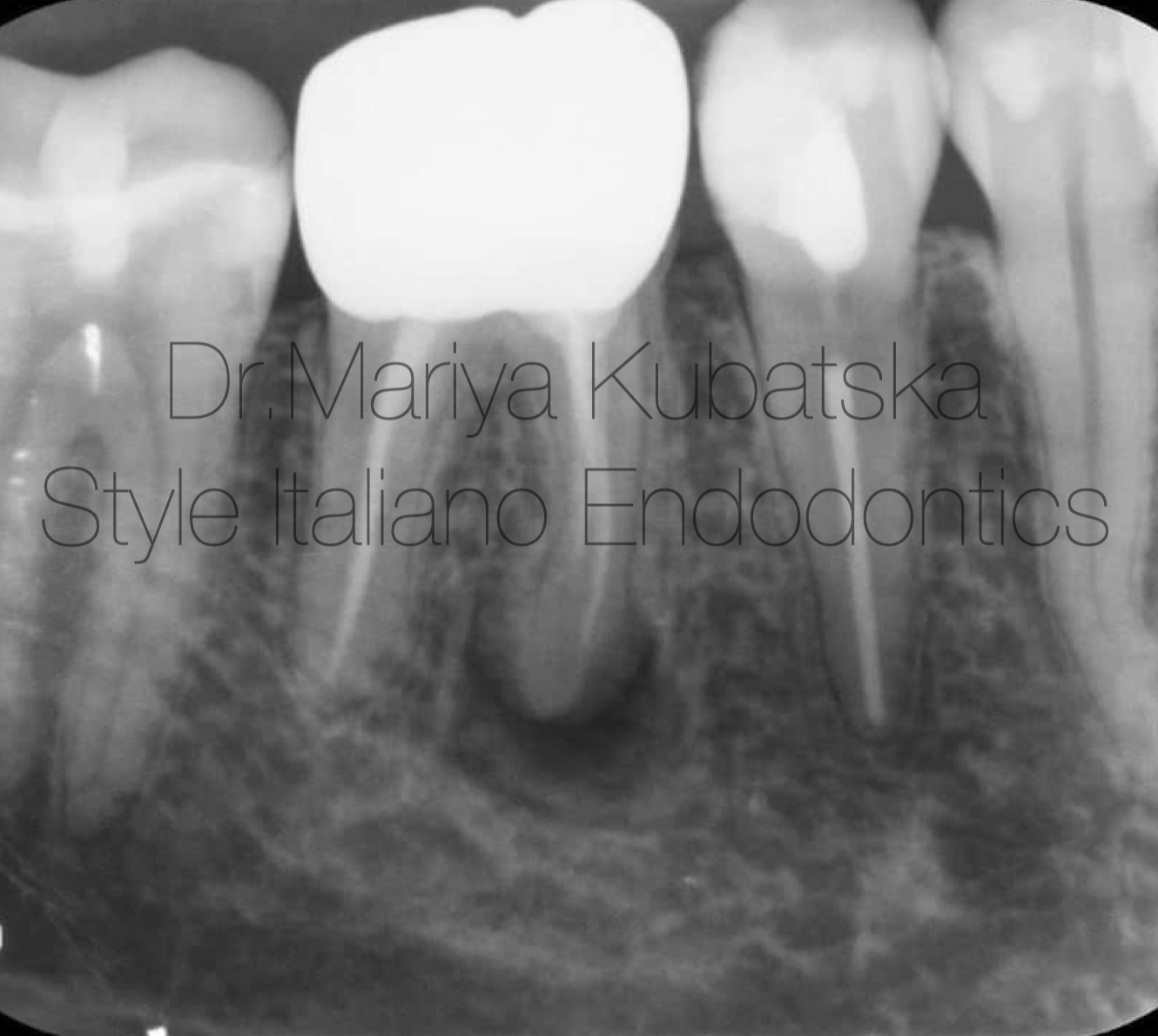
Fig. 1
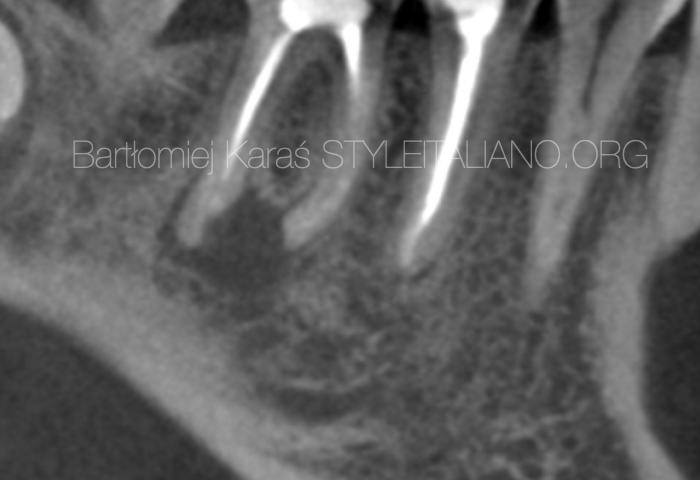
A 40-year-old patient was referred to me for non-surgical re-treatment of tooth 46. On clinical examination, a zirkonium crown was detected. No signs of crack or fracture. The probing depth was no more than 3 mm. No pain on percussion or palpation. No mobility. Diagnosis and pulp status: Previously treated, asymptomatic apical periodontitis. Pre-opp RVG showed the presence of apical lesion and that mesial root canals were not filled correctly.
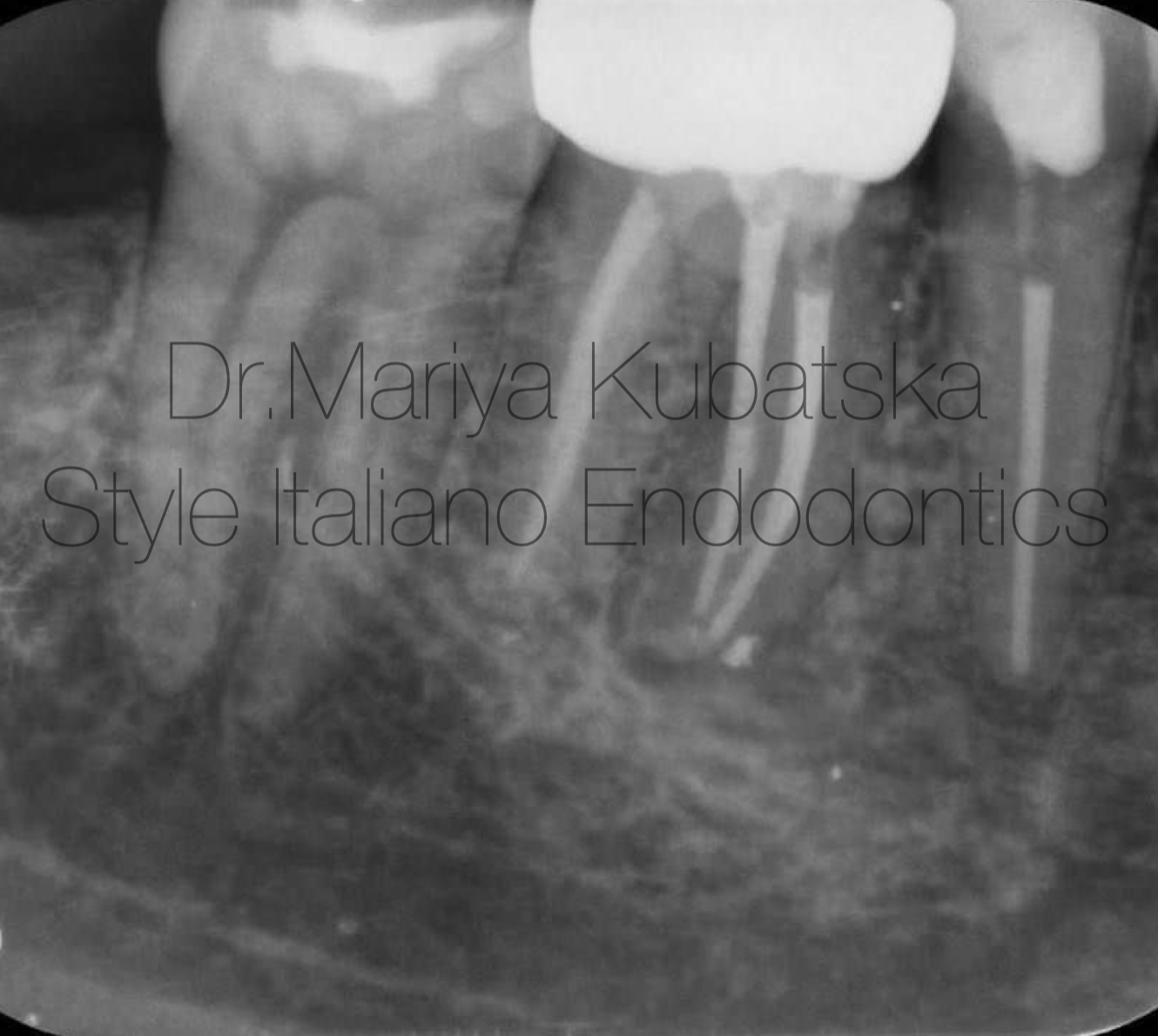
Fig. 2
It was performed 1 visit re-treatment. Gutta-percha was removed mechanically using instruments Endostar Re-Endo Rotary System, and Orange Guttane. ML, MB, and D root canals were instrumented to ISO 30.06. Endodontic solutions were activated by EDDY,PUI. Obturation was made with epoxy sealer and gutta-percha by using a hybrid technique. One-year recall: At the 12 months recall, RVG evaluation showed complete healing of periapical bone.
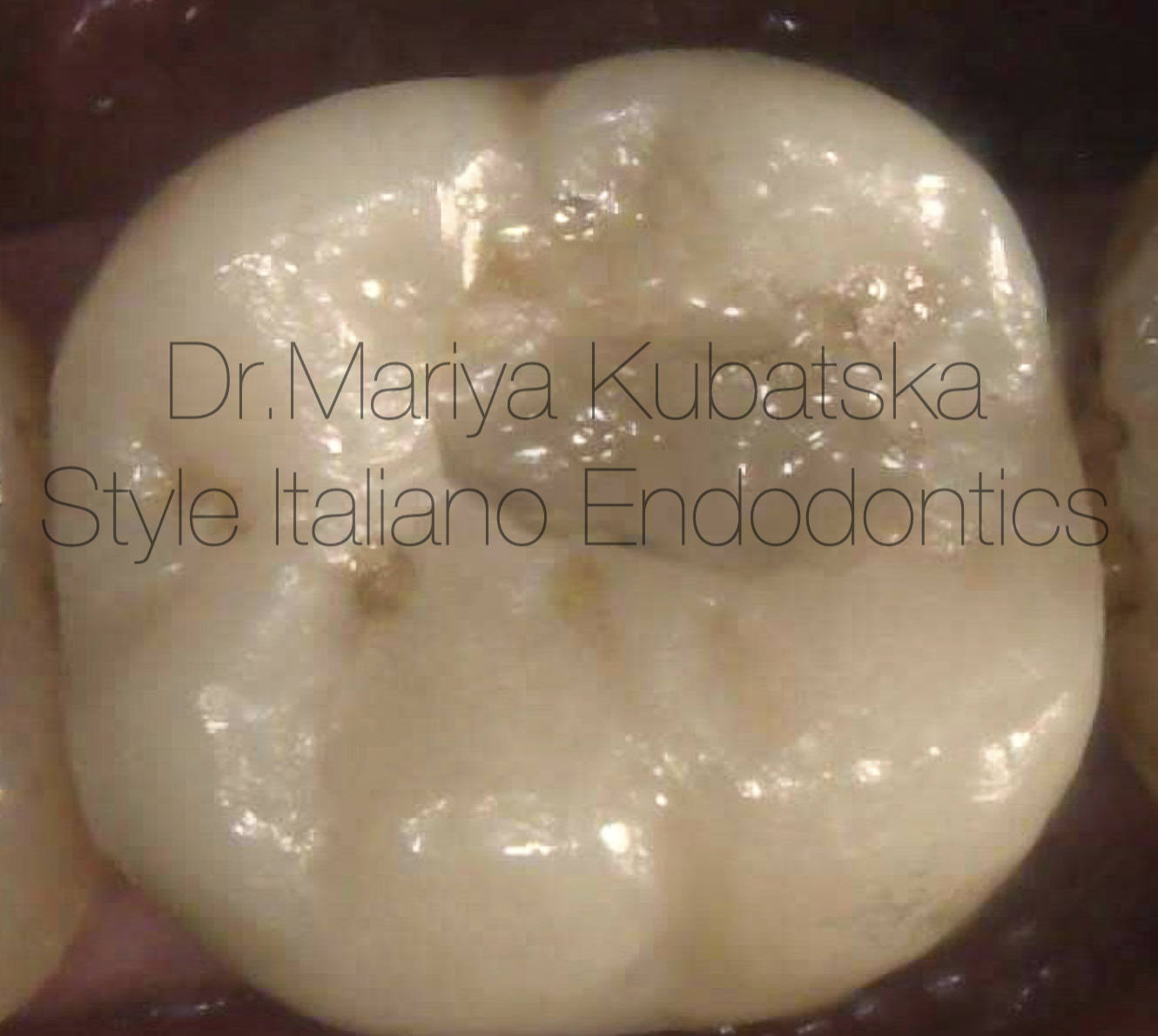
Fig. 3
The appearance of a prothetic crown after minimal invasive access and filling with composite after root canal treatment.
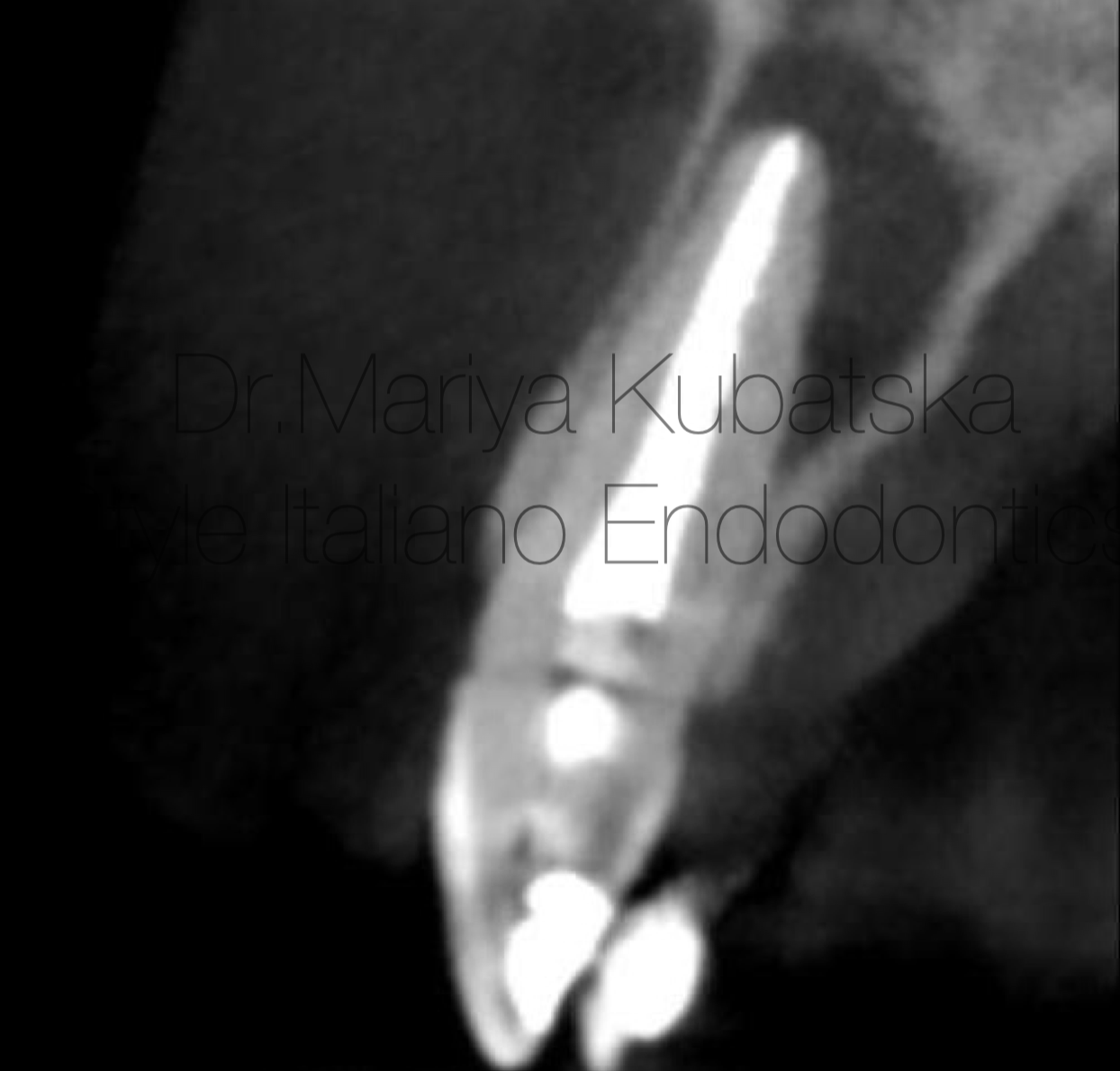
Fig. 4
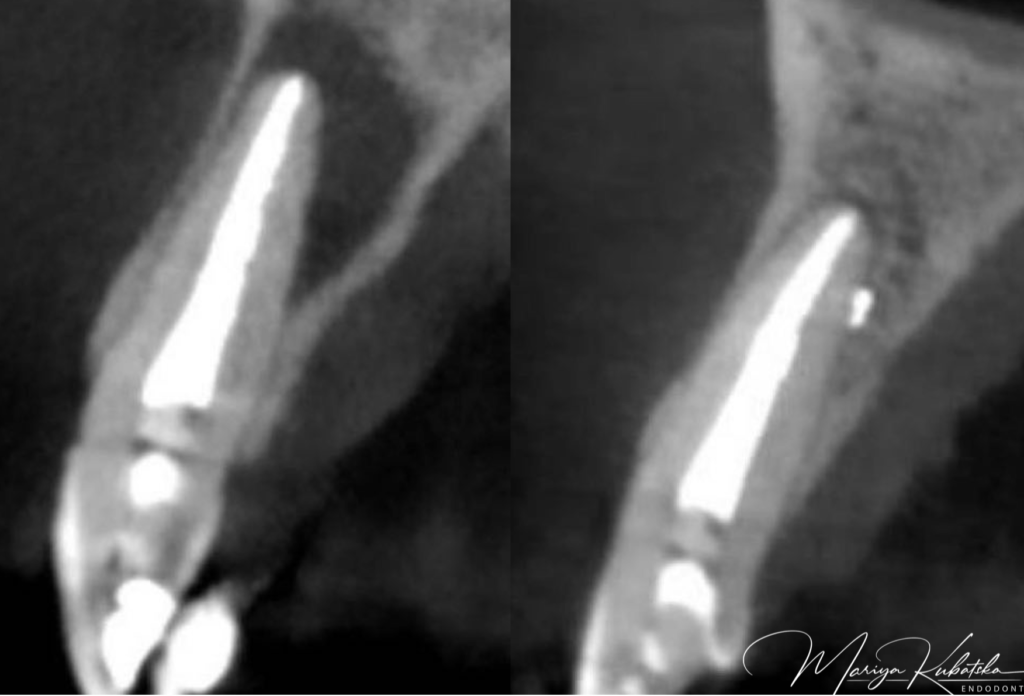
A 30-year-old patient was referred to me for non-surgical re-treatment of tooth 22. On clinical examination, the composite filling was detected. No signs of crack or fracture. The probing depth was no more than 3 mm. No pain on percussion or palpation. No mobility. Diagnosis and pulp status: Previously treated, asymptomatic apical periodontitis. Pre-opp CBCT, Sagittal view showed the presence of an apical lesion, tooth 22-previously treated.
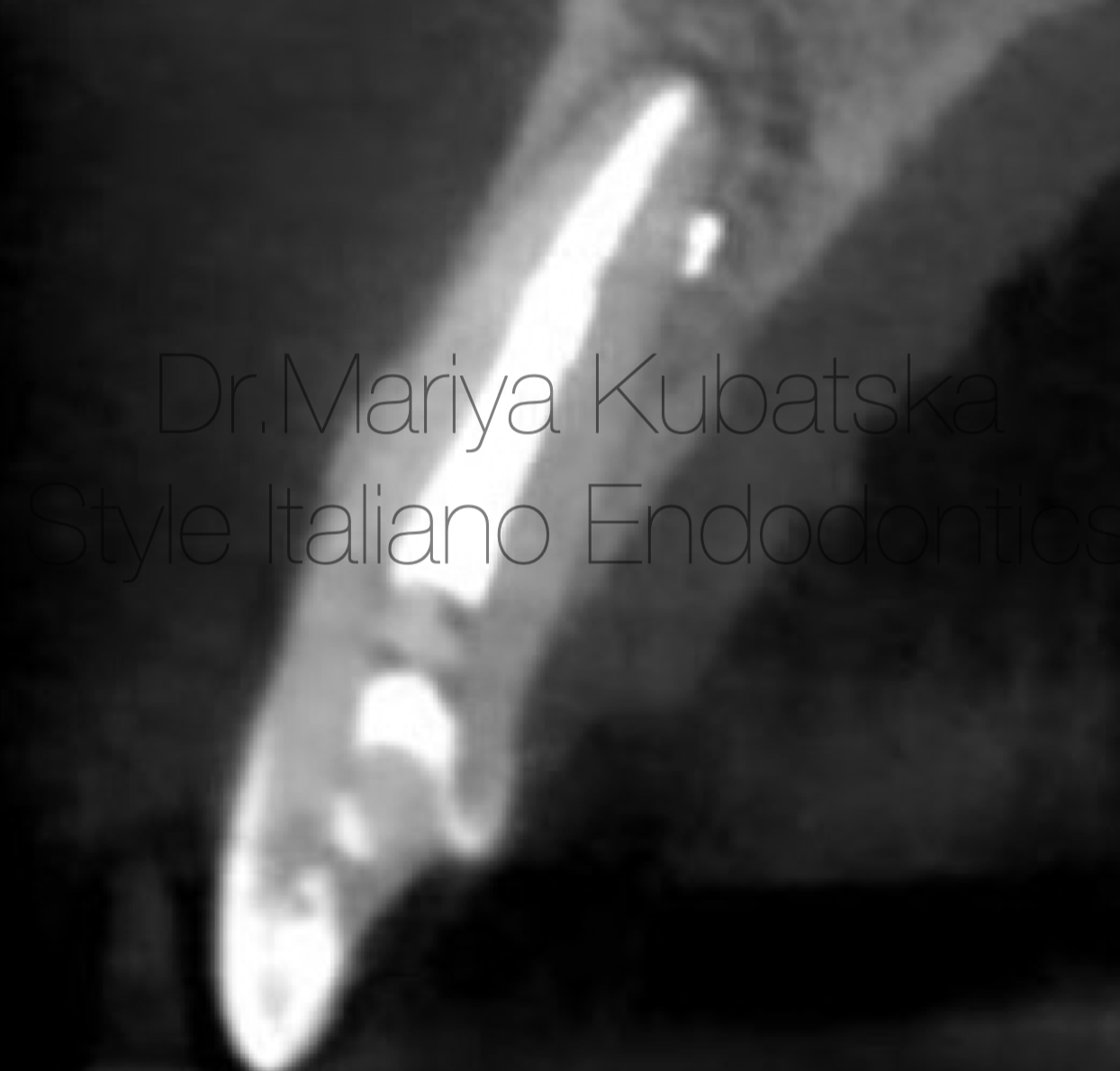
Fig. 5
It was performed 1 visit re-treatment. Gutta-percha was removed mechanically using instruments Endostar Re-Endo Rotary System, and Orange Guttane. Root canal was instrumented to ISO 45.04. Endodontic solutions were activated by EDDY, PUI. Obturation was made with epoxy sealer and gutta-percha by using a CWT technique. At the 12 months recall, СBCT evaluation sagittal view showed complete healing of periapical bone.
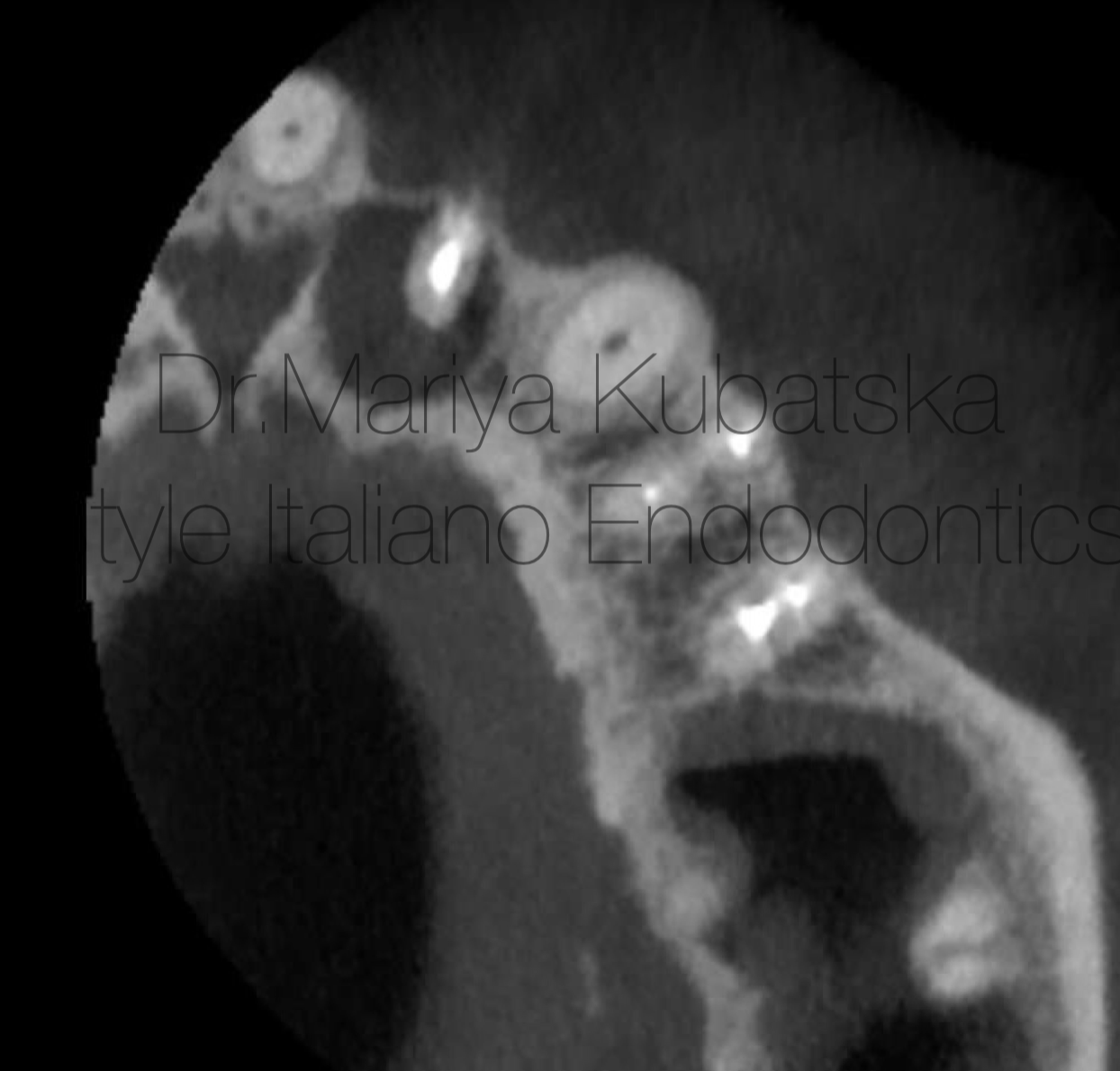
Fig. 6
Pre-op CBCT of the same patient and the same tooth 22, axial view showed the presence of an apical lesion, teeth 22,24,25 previously treated.
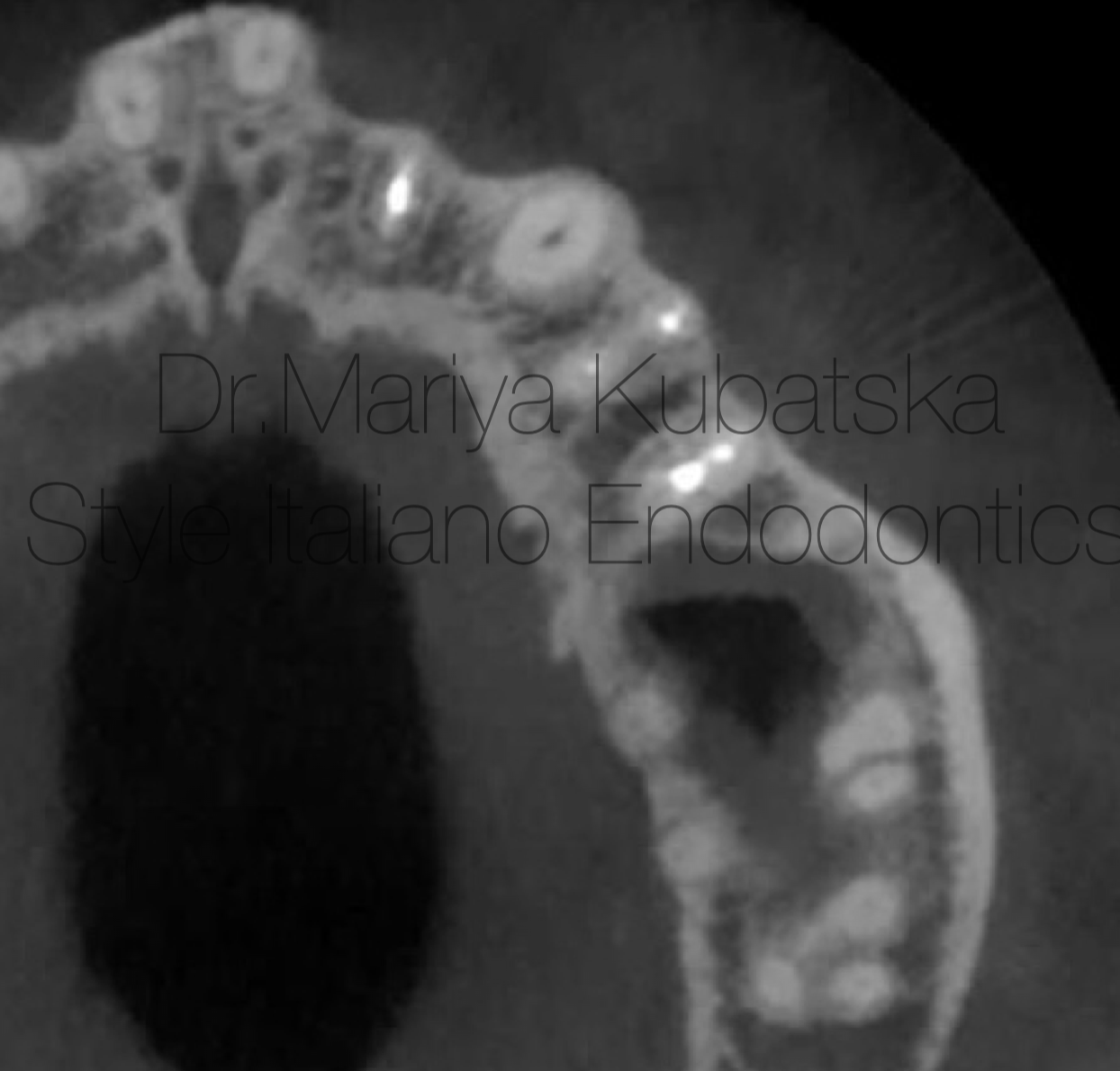
Fig. 7
12 months recall, СBCT evaluation axial view showed complete healing of periapical bone.
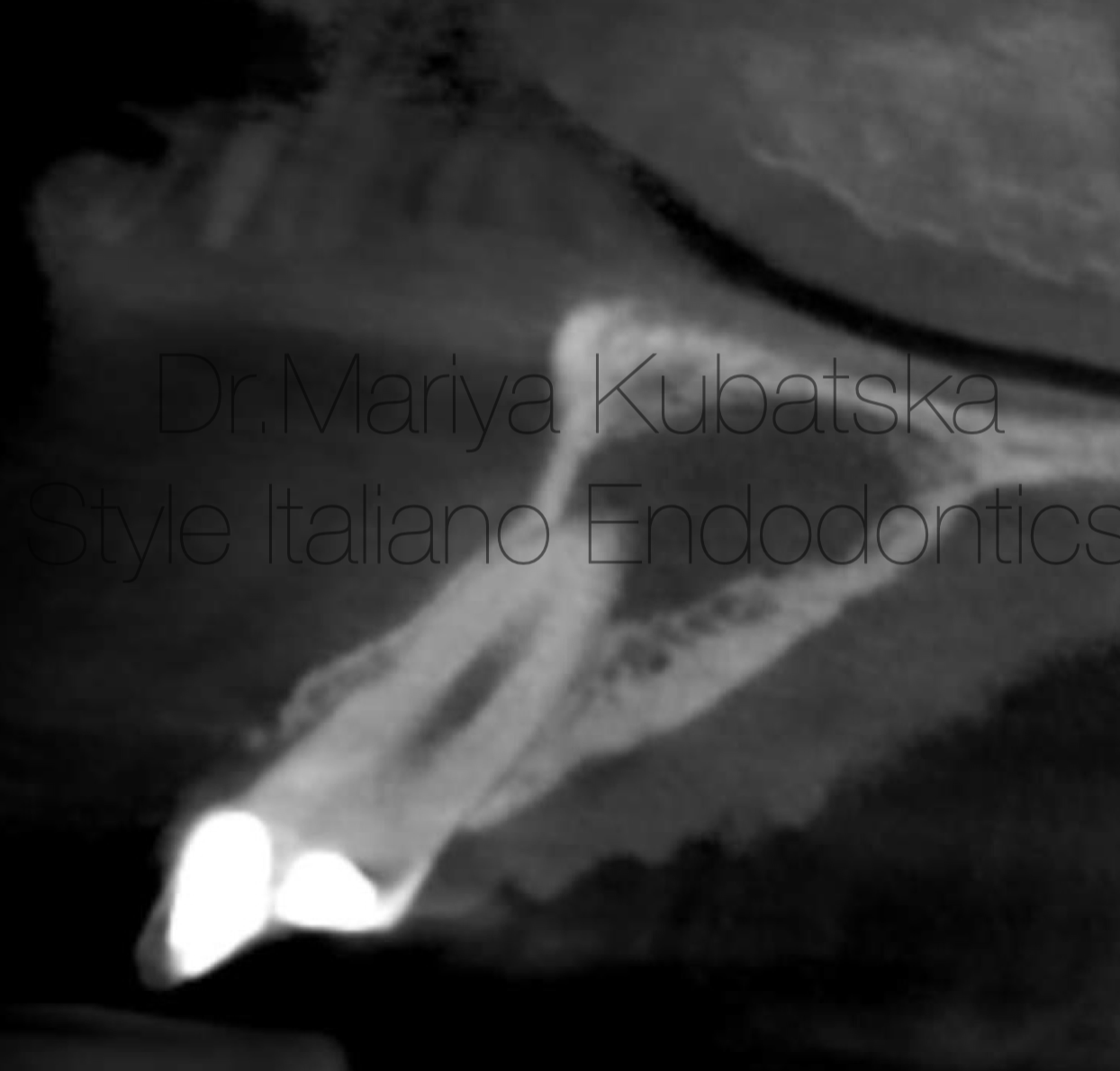
Fig. 8
A 18-year-old patient was referred to me for non-surgical treatment of tooth 12. On clinical examination, the composite filling was detected. No signs of crack or fracture. The probing depth was no more than 3 mm. Pain on percussion, pain on palpation. No mobility. Diagnosis and pulp status: necrosis, asymptomatic apical periodontitis. Pre-opp CBCT, Sagittal view showed the presence of an apical lesion.
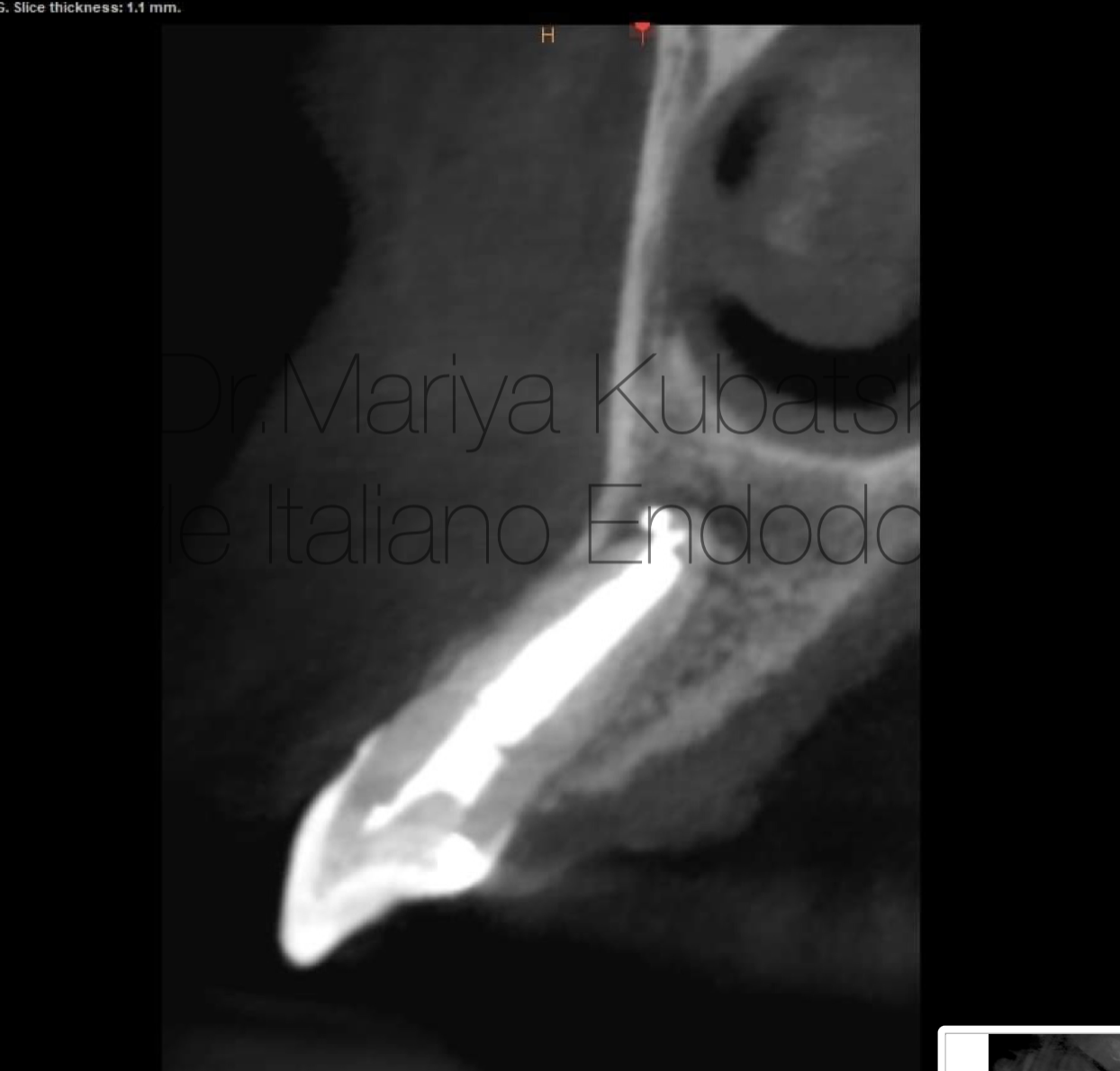
Fig. 9
It was performed 2 visits root canal treatment. Root canal was instrumented to ISO 50.04 according to apical gauging. Endodontic solutions were activated by EDDY, PUI. Obturation was made with bioceramic sealer putty and MAP system PD Dental. At the 12 months recall, СBCT evaluation sagittal view showed complete healing of periapical bone.
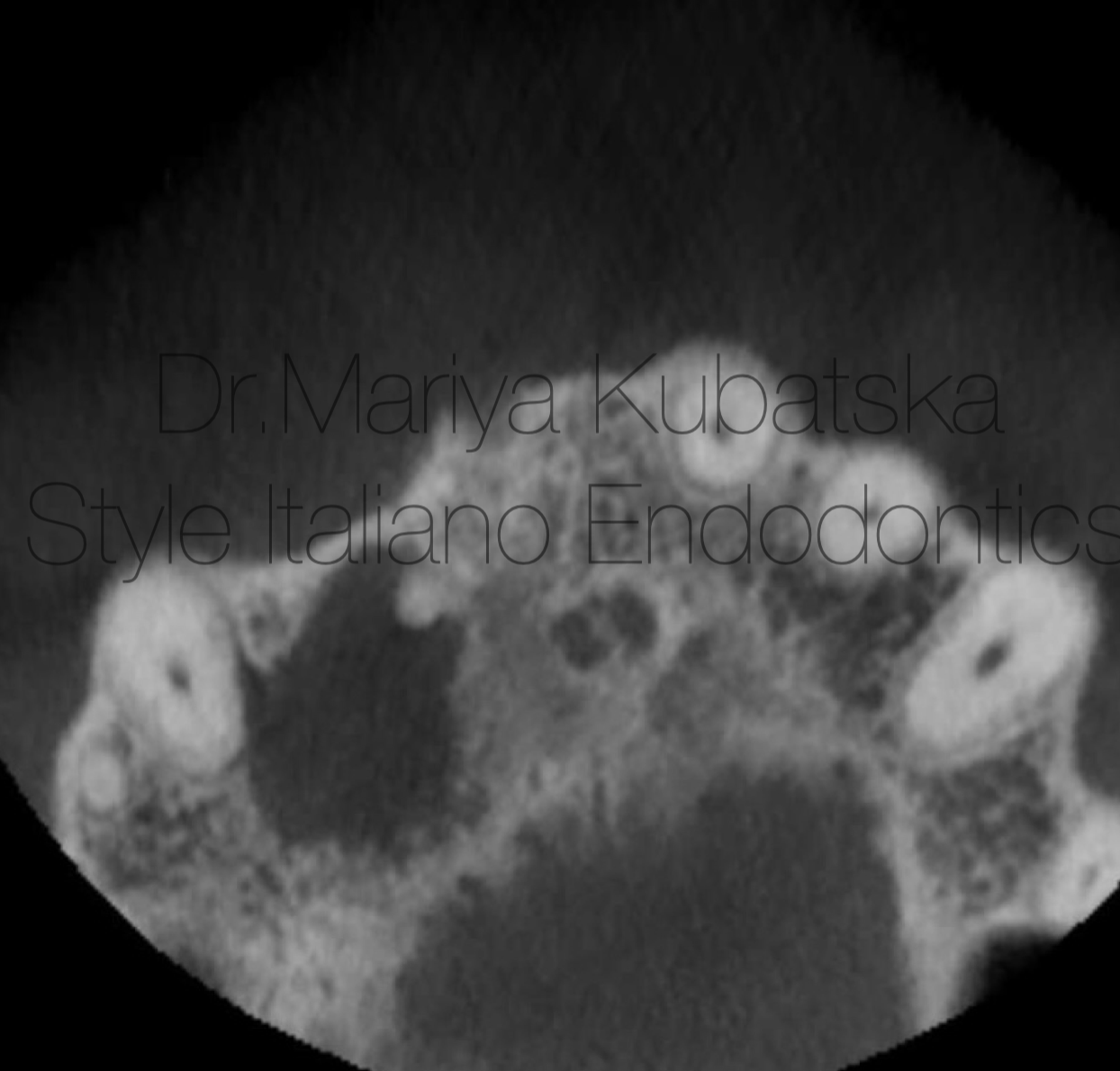
Fig. 10
Pre-opp CBCT of the same patient and the same tooth 12, axial view showed the presence of an apical lesion.
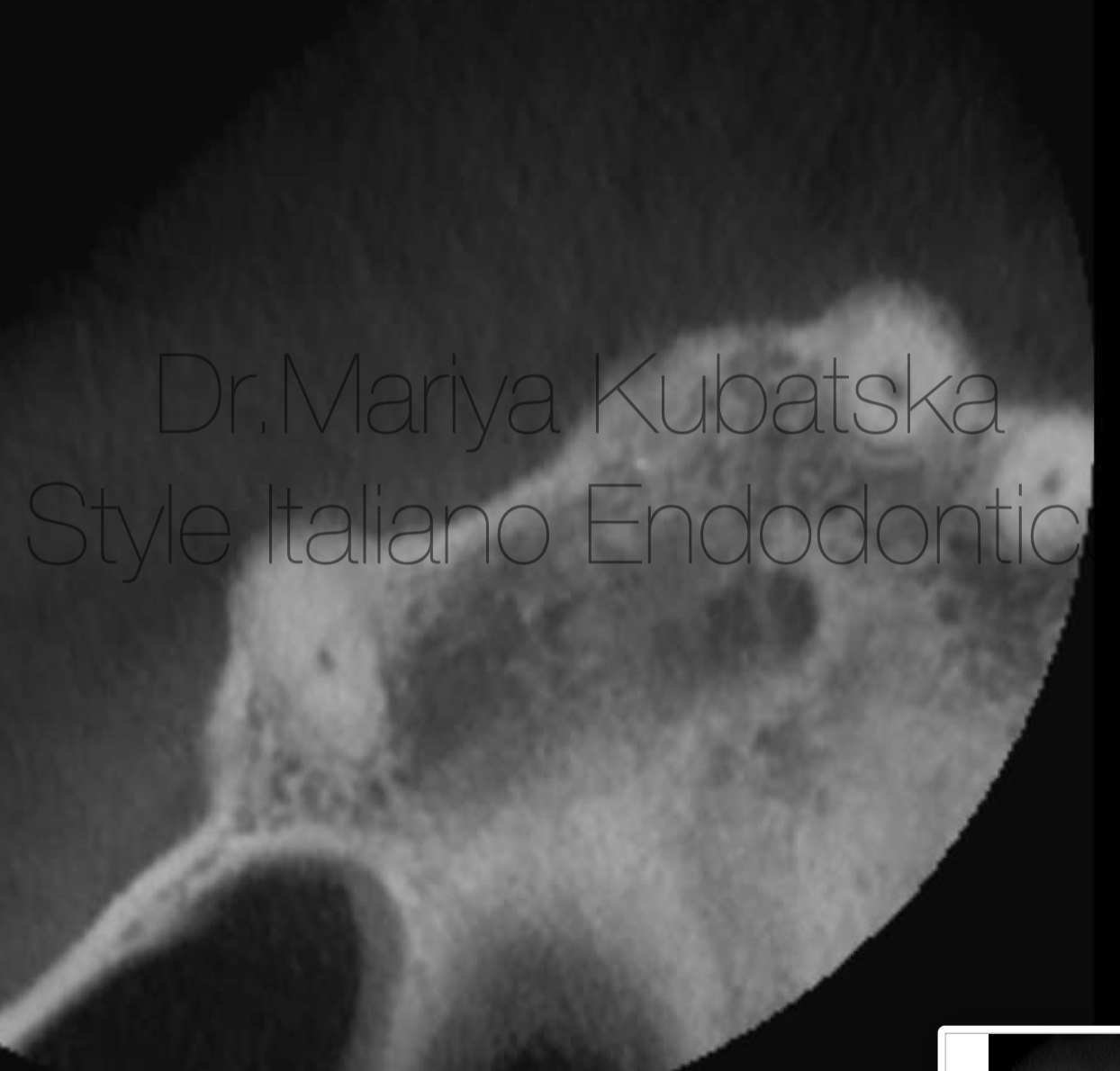
Fig. 11
12 months recall, СBCT evaluation axial view showed complete healing of periapical bone.
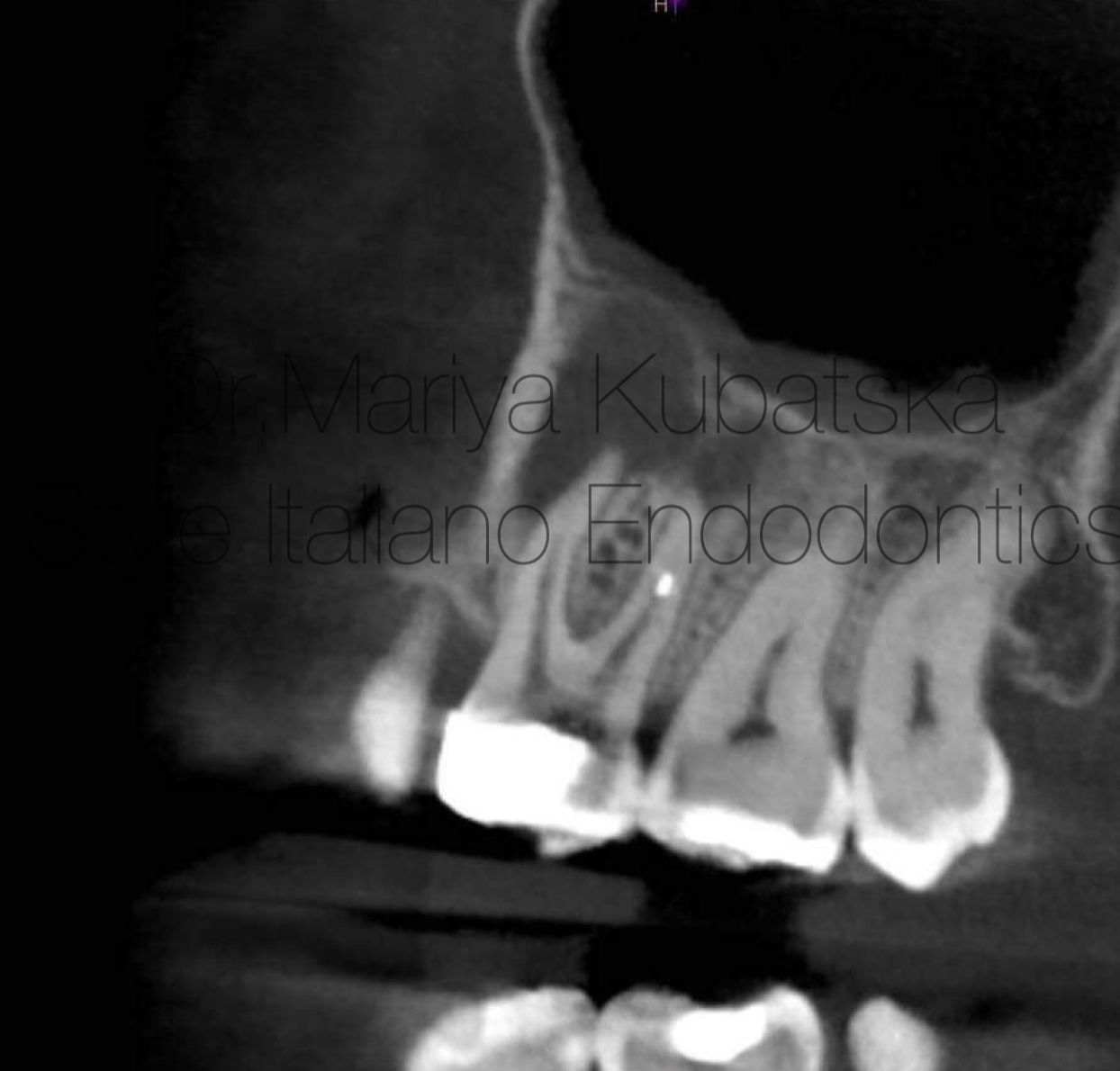
Fig. 12
A 40-year-old patient was referred to me for non-surgical treatment and broken file removal tooth 16. On clinical examination, a composite filling was detected. No signs of crack or fracture. The probing depth was no more than 3 mm. No pain on percussion or palpation. No mobility. Diagnosis and pulp status: necrosis, asymptomatic apical periodontitis. Pre-opp CBCT coronal view showed the presence of apical lesion and separated instrument in DB root canal.
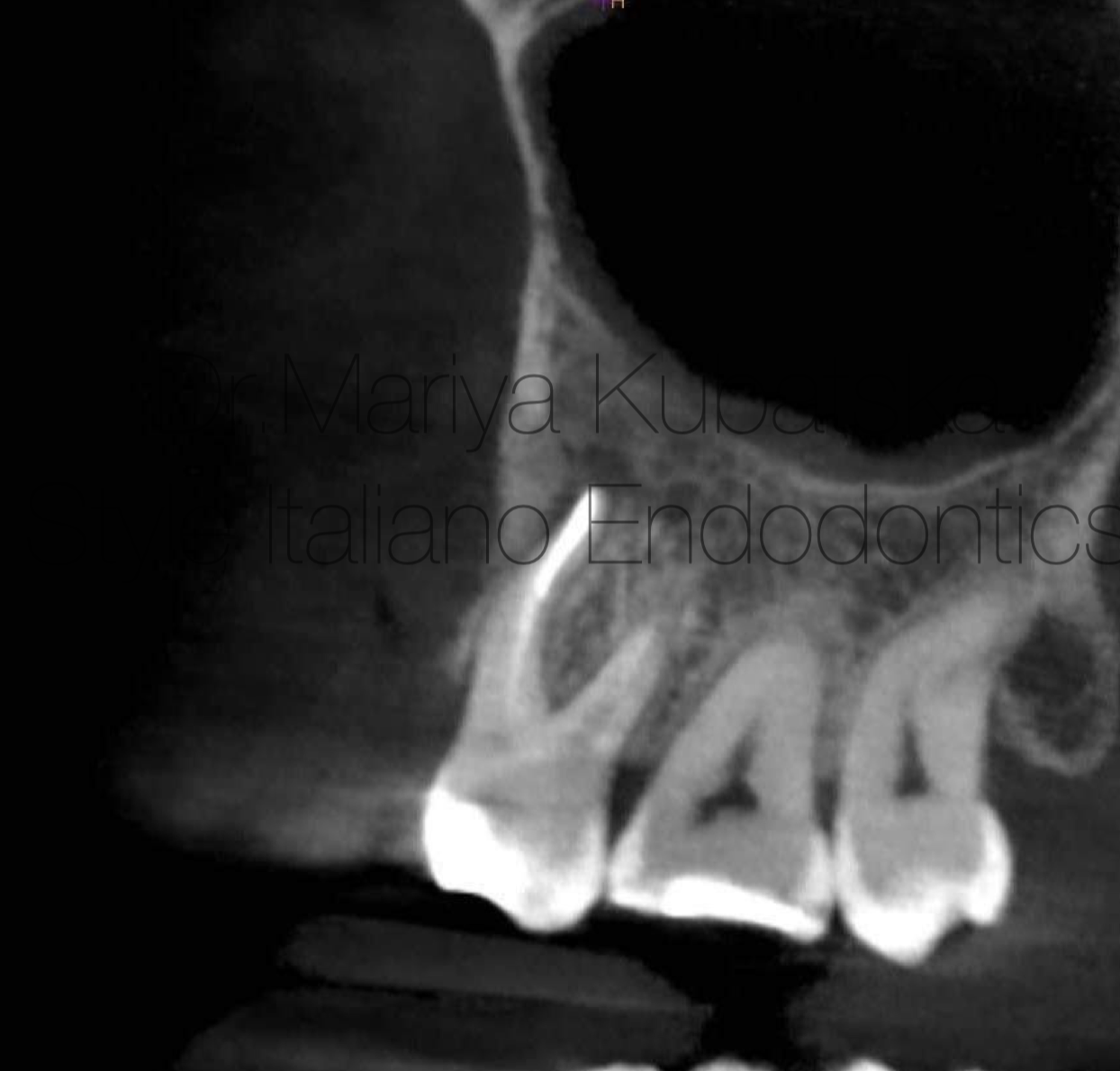
Fig. 13
It was performed 2 visits root canal treatment. MB1,MB2,DB root canals were instrumented to ISO 30.06, broken file was removed by using Ultrasonics technique, P root canal was instrumented to ISO 40.06. Endodontic solutions were activated by EDDY, PUI. Obturation was made with epoxy sealer and gutta-percha by using CWT technique. At the 12 months recall, СBCT evaluation sagittal view showed advanced healing of periapical bone.
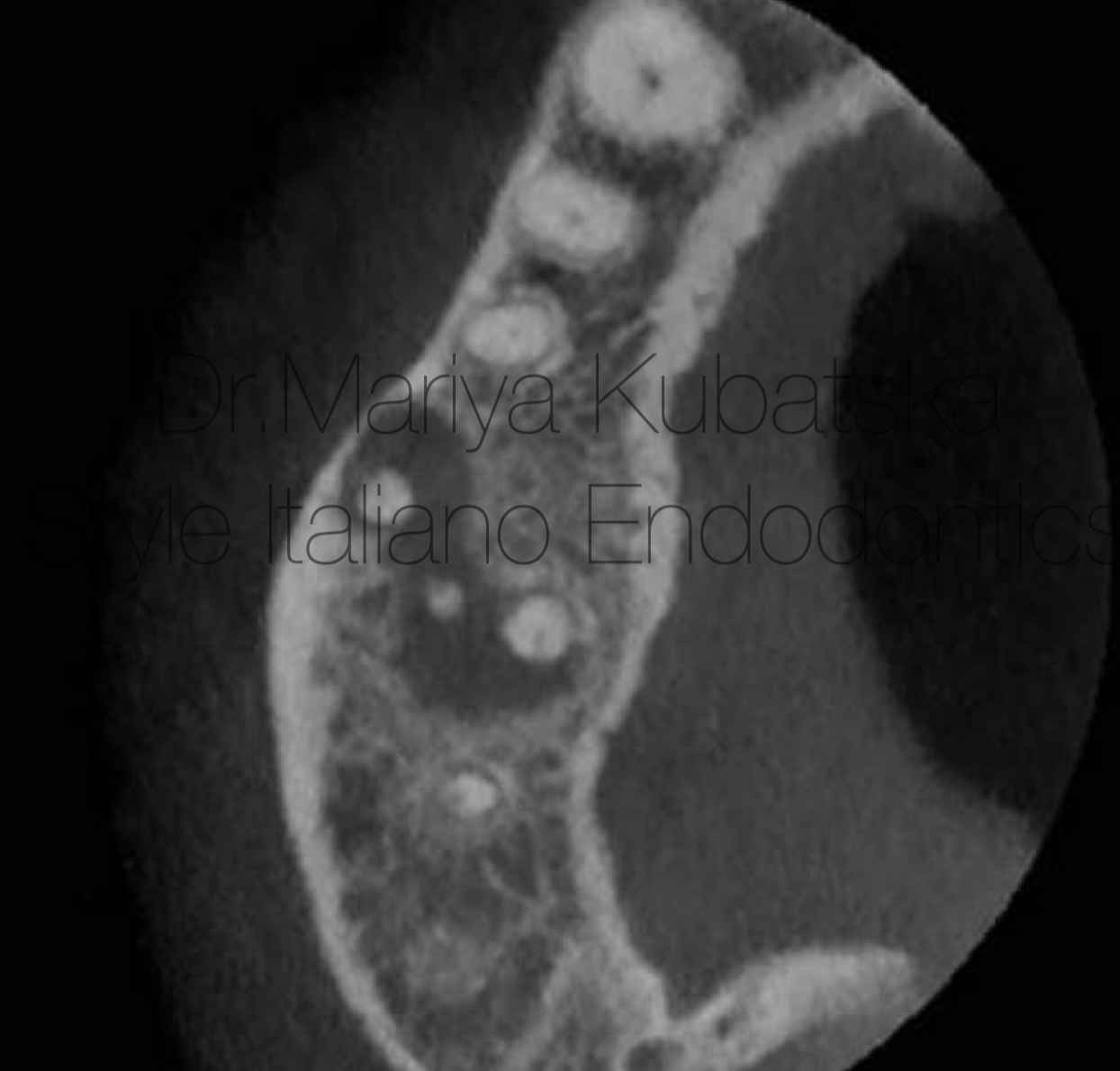
Fig. 14
Pre-opp CBCT of the same patient and the same tooth 16, axial view showed the presence of an apical lesion.
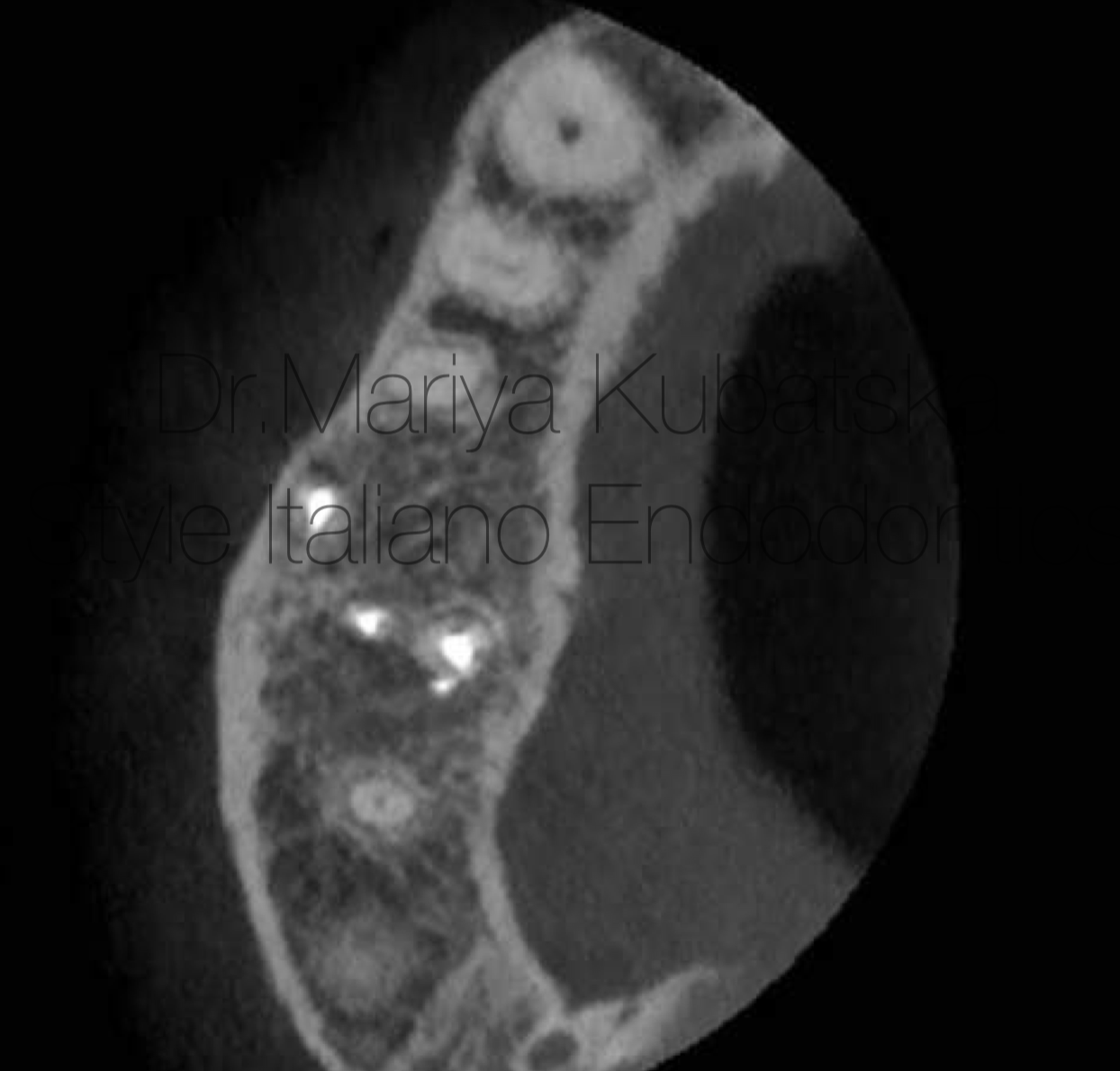
Fig. 15
12 months recall, СBCT evaluation axial view showed advanced healing of periapical bone.

Fig. 1
About the author:
Mariya Kubatska graduated medical university of Gdansk in 2014. Since graduation highly interested in Endodontics. Member of the European Society of Endodontology, the American Association of Endodontists, member of Polish Endodontic Association, and the Department of Endodontology of the Polish Dental Society. Since 2023 Style Italiano Endodontics fellow. International speaker. Lecturer in Esdent Dental Training Company. Author of case reports in Polish dental magazines. Since 2018 Mariya has worked in Oslo, Norway, focusing on endodontics. In private life piano lover.
Conclusions
Apical lesions play a protective role by inhibiting bacterial spread. Understanding the mechanisms of lesion formation and the factors influencing lesion healing is crucial in managing this condition. Proper disinfection of the root canal system leads to the elimination of the infection source and is a key to healing, which is a success of endodontic treatment.
Bibliography
- Ørstavik, D. (1996) Time-course and risk analyses of the development and healing of chronic apical periodontitis in man. International Endodontic Journal, 29, 150–155.
2.Siqueira, J.F. Jr., Rôças, I.N., Riche, F.N.S.J. & Provenzano, J.C. (2008) Clinical outcome of the endodontic treatment of teeth with apical periodontitis using an antimicrobial protocol. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics, 106, 757–762.
3.Friedman, S. (2002) Prognosis of initial endodontic therapy. Endodontic Topics, 2, 59–88.
4.Ørstavik, D., Qvist, V. & Stoltze, K. (2004) A multivariate analysis of the outcome of endodontic treatment. European Journal of Oral Sciences, 112, 224–230.
5.Metzger, Z., Lin, Y., DiMeo, F., Ambrose, W., Trope, M. & Arnold, R.R. (2009) Synergistic pathogenicity of Porphyromonas gingivalis and Fusobacterium nucleatum in the mouse subcutaneous chamber model. Journal of Endodontics, 35, 86–94.
6.Genco, C.A., Cutler, C.W., Kapczynski, D., Maloney, K. & Arnold, R.R. (1991) A novel mouse model to study the virulence of and host response to Porphyromonas (Bacteroides) gingivalis. Infection and Immunity, 59, 1255–1263.
7.Metzger, Z. (2000) Macrophages in periapical lesions. Endodontics and Dental Traumatology, 16, 1–8.
8.Metzger, Z., Lin, Y., DiMeo, F., Ambrose, W., Trope, M. & Arnold, R.R. (2009) Synergistic pathogenicity of Porphyromonas gingivalis and Fusobacterium nucleatum in the mouse subcutaneous chamber model.
9.Metzger, Z., Abramovitz, I. & Bergenholtz, G. (2009) Apical periodontitis. In: Bergenholtz, G., Horsted-Bindslev, P. & Reit, C. (eds), Textbook of Endodontology, 2 edn. Wiley-Blackwell Munksgaard, Chichester, UK, pp. 113–127.
10.Essential Endodontology, Prevention and Treatment of Apical Periodontitis edited by Dag Ørstavik.