System-Based Endodontics: does your gutta percha master cone fit?
06/10/2017
Cliff Ruddle
Warning: Undefined variable $post in /var/www/vhosts/styleitaliano-endodontics.org/endodontics.styleitaliano.org/wp-content/plugins/oxygen/component-framework/components/classes/code-block.class.php(133) : eval()'d code on line 2
Warning: Attempt to read property "ID" on null in /var/www/vhosts/styleitaliano-endodontics.org/endodontics.styleitaliano.org/wp-content/plugins/oxygen/component-framework/components/classes/code-block.class.php(133) : eval()'d code on line 2
Allow me to describe a clinical dilemma that frequently happens when performing endodontic treatment. Let’s say you have experience, are well trained, and have just finished cutting a fabulous endodontic access cavity preparation in accordance with your philosophy of treatment. You identify all of the orifices on the pulpal floor of this multi-rooted tooth. You initiate glide path management procedures and successfully negotiate, catheterize, and secure any given canal. You carry your favorite mechanical shaping file to the full working length, then remove it, and note its apical flutes are loaded with dentine mud. You are pleased, as this visual observation confirms this file just cut its shape in the apical one-third of this particular canal.
You recall the check-off list of procedural questions, such as “When are you ready to pack?” Answer: when you can fit the gutta percha master cone (GPMC). “When can you fit the GPMC?” Anwer: when you have the shape. “When do you have the shape?” Answer: when the apical flutes of the last shaping file carried to the full working length are loaded with dentine mud. Although you recognize 3D disinfection and filling root canal systems are the next procedural clinical steps, you first select a system-based GPMC to reconfirm the shape. Sure enough, this cone and a few more same-sized cones do not fit. You note that some cones fit short of the desired working length, or are distorted terminally, or slide long.
Your frustration mounts as you begin to doubt if there is even one single GPMC in the entire box of cones that will slide to length and exhibit apical tug back. Regrettably, when a GPMC does not fit, some clinicians assume there is a shaping problem and begin to needlessly modify this already ideal shape. System-based endodontics is a well-intended concept that implies there is a sizing inter-relationship between or among specifically sized mechanical files, GPMCs, and paper points (Figure 1). The clinical expectation is that it should be effortless to find a GPMC that will accurately correspond to the size of the largest manual or mechanical shaping file carried to the full working length.
System-based endodontics is designed to simplify decision-making, encourage efficiency, and promote accuracy. However, the bad news is system-based endodontics has largely failed to provide a good clinical working precision between and among various products. There is an old expression that a problem recognized is a problem half solved; as such, recognizing the GPMC sizing and formulation dilemma has created a breakdown to breakthrough moment in clinical endodontics. System-based endodontics is no longer a theoretical concept; rather, system-based endodontics has emerged and represents what you are looking for that is frequently missing.
FINDING THE IDEAL FILLIN MATERIAL
More than 70 years ago, Prof. Louis Grossman identified the requirements for an ideal root canal filling material [1]. In essence, this ideal material should seal any given canal and its related root canal system, and be dimensionally stable and biologically inert. Additionally, the endodontic filling material should be radiopaque, not stain tooth structure, and should be sized to promote the concept of system-based endodontics. Finally, any given obturation material should be readily removable in the instance of nonsurgical retreatment. Importantly, with the growing emphasis on minimally invasive endodontics, future obturation materials and methods must also fulfill the Grossman requirements for an ideal root canal filling material.
Due to the complexity of endodontic anatomy, many materials, technologies, and methods have been advocated for filling root canal systems (Figure 2). Although gutta percha was introduced in dentistry in the mid-1800s, in the past decade, considerable interest has focused on improving traditional GPMCs, along with existing materials, such as resins, glass ionomers, bioceramics, bioglass, silicon-based formulations, and MTA-based sealers. These new generational products have been engineered and formulated in the hope of filling root canal systems better, easier, and faster. With all this effort, little emphasis has focused on how to technologically exploit gutta percha, or trans-1,4 polyisoprene (TPI), and unlock its full potential.
FILLING METHODS
A great variety of methods are available for filling root canal systems. Except for carrier-based obturators or injection-filling techniques, virtually all other obturation methods involve fitting a specifically sized and formulated GPMC. For example, a GPMC is fit into a prepared canal in order to use cold lateral condensation, warm gutta percha with vertical condensation, continuous wave, or a thermomechanical, single cone, or hybrid method (Figure 3). Additionally, a GPMC can be further adapted to the preparation using a more desirable cold rolling vs. an undesirable chemical method. However, there are critical distinctions between the myth of traditional system-based GPMCs vs. the new system-based, state-of-the-art GPMCs.
TRADITIONAL GPMCs
Traditional GPMCs have long been produced using slave-like hand-rolling labor (Figure 4). However, in recent years, producers have begun using machine-rolling methods to improve accuracy and efficiency. Regardless of the manufacturing pathway, both methods produce a more problematic fixed-tapered GPMC. Fixed tapered GPMCs frequently have a rate of taper that exceeds the rate of taper of the final preparation. This means these cones oftentimes bind in the body of the canal, fit loose at length, and clinically exhibit false apical tugback. Additionally, fitting a fixed-tapered GPMC into virtually any final preparation can be clinically challenging, as many shaping files are currently designed to cut more conservative shapes in the body of the canal; or, clinicians are choosing to cut smaller tapered preparations with greater emphasis on the concept of minimally invasive endodontics.
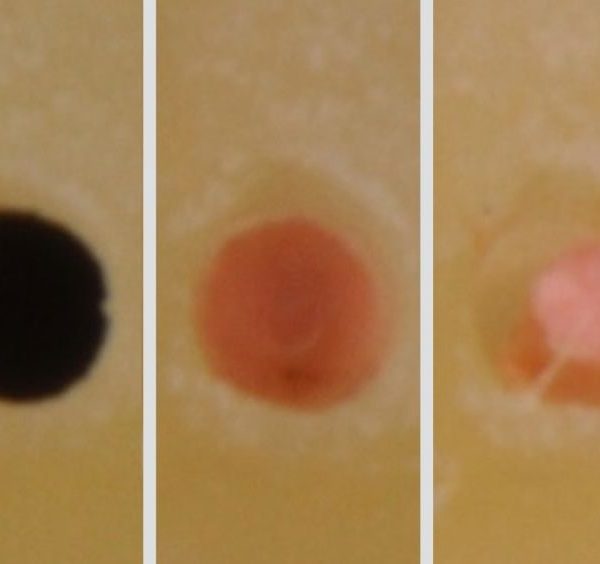
In addition to the fixed-tapered cone issue is the fact that manufacturers are only required to produce GPMCs to an ISO regulated tolerance of ±0.05 mm. Clinically attempting to fit any cone with this super loose tolerance is an annoyance that also contributes to cone fit difficulties (Figure 5). As a clinical example, when the final shaping file carried to length is a size 30/06, then the clinician typically selects a system-based correspondingly-sized GPMC. However, in accordance with ISO tolerances, this GPMC could have a tip diameter anywhere between 0.25 mm and 0.35 mm. Clinicians have long noted the discrepancies in terminal diameters and tapers between and among same-sized GPMCs in the same box (Figure 6).
Another deficiency with traditional GPMCs is related to formulation. To review, the traditional gutta percha formulation is, more or less, 18%-22% TPI, about 70% zinc oxide (ZnO) filler, a heavy metal barium sulfate opacifier, and a coloring agent, such as Canada balsam. This low percent of TPI means a higher working temperature is required. Compounding the formulation problem is the undesirable introduction of waxes utilized to improve flow characteristics. In summary, using higher working temperatures in combination with waxes invites material breakdown, where the filler becomes unbound and leaches out of the formulation, predisposing to inflammatory reactions.
Traditionally sold GPMCs can carry a maximum heat wave of 3-4 mm. Yet, Schilder’s research validates a 5 mm heat wave attributable to thermal cycling when using the classic warm gutta percha with vertical condensation technique (Figure 7) [2-3]. Further, Schilder’s research shows that a GPMC elevated 3ºC above body temperature becomes sufficiently thermosoftened and can be readily molded into the preparation at temperatures between 40º and 45ºC [4-5]. However, producing this heat wave to the terminus of the master cone can be limiting when anatomy restricts placing an electric heat carrier and working pluggers in longer, narrower, and more curved canals, whether minimally or fully prepared.
STATE-OF-THE-ART GPMCs
Healthdent Technology International was established in 1996 by Dr. Nathan Li, a practicing dentist, to manufacture high quality, innovative nano synthetic GPMCs, pellets, cartridges, and delivery systems for filling root canal systems. In 2015, after many years of R&D, Healthdent launched new, synthetic system-based GPMCs that provide superior sizing and formulation. Additionally, Healthdent developed vastly improved injectable synthetic nano flow gutta percha that has exceptional handling characteristics tightly regulated by a formulation that allows a significantly lower working temperature. Dentists and patients, alike, are benefitting from Healthdent’s commitment to develop innovative and affordable endodontic technologies.
Specifically, Healthdent recognized that, in order for clinical endodontics to move ever closer toward a true system-based reality, the GPMC dilemma must be resolved. As such, Healthdent began fabricating precision mold cavities using electro-discharge machining (EDM) vs. utilizing less precise grinding wheels. In this manner, an electrical spark, in an acid medium, can remove as little as 0.00254 mm of mold material per pass. This innovative process has allowed GPMCs to be produced that have tolerances of ±0.02 mm, or the same tolerances ISO requires of manufacturers that produce shaping files (Figure 8). EDM has enabled the production of both fixed-tapered and multi-tapered GPMCs that precisely correspond to files that have either a fixed or variably tapered design over their active portion. A fixed- or multi-tapered cone with superb sizing tolerances has led to a predictable cone fit virtually every single time (Figure 9).
Another major breakthrough in synthetic gutta percha (TPI) formulation is related to using an acid bath during kneading to purify and loosen the molecular chain. This loosening process allows the addition of heat conductive nano particles, which extend the linear heat wave along the master cone from 3mm to 6 mm, without the benefit of thermo-cycling. Strategically, increasing the TPI ratio, compared to traditional formulations, allows for using substantially lower working temperatures, which equates to less volumetric changes and increased material density. Gutta percha with nano formulation technology is also a better polymer matrix to incorporate so-called bio-regenerative materials, such as MTA and bioceramics, and will further integrate the interface between GPMCs and emerging bio-regenerative sealers. Lastly, these formulation benefits are driving the development of new miniaturized – and hence, ergonomic - cordless, motorless, and heatless delivery technologies.
With the gutta percha improvements in sizing and formulation, fitting a system-based GPMC into a well-prepared canal and filling root canal systems has never been easier. Analogous to the dental impression, thermosoftened gutta percha is the heavy-bodied material that can be readily molded and adapted to the internal configuration of the prepared canal with a pre-fit plugger. In turn, this so-called heavy-bodied material hydraulically drives the light-bodied material, or root canal sealer, into all aspects of the root system (Figure 10). In this manner, the sealer interface between gutta percha and dentin has been measured, and is on the order of 7, 8, or 9 microns (µm) [6]. Although gutta percha creates the “piston effect,” it is well known that it is the sealer that actually seals the root canal system (Figure 11) [7].
Today, Healthdent produces precisely sized and superbly formulated GPMCs that correspond to virtually any distributor’s final shaping file that is carried to the full working length. Several dental companies, such as Charles B. Schwed, Obtura Spartan, and Dentsply Sirona, distribute these unique GPMCs, pellets, and injectable cartridge-based gutta percha products.
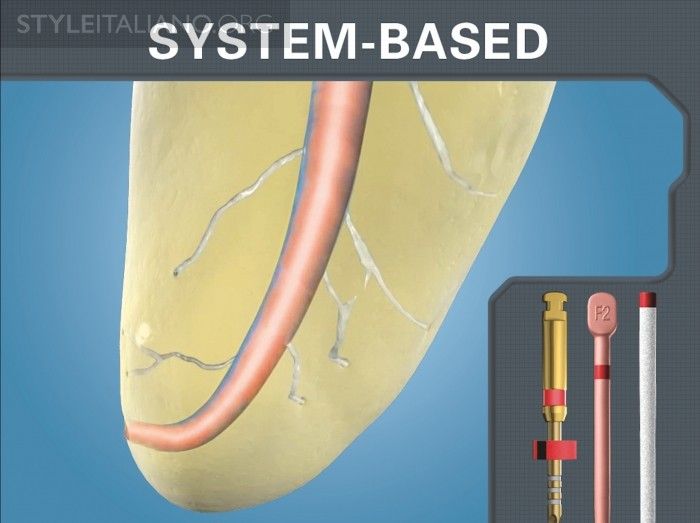
Fig. 1
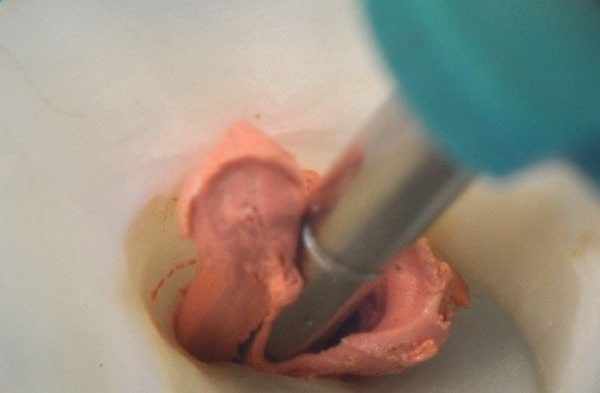
Img. 1 - This image demonstrates a nano synthetic, multi-tapered, system-based GPMC that has a rate of taper less than the final preparation and is snug only at length.
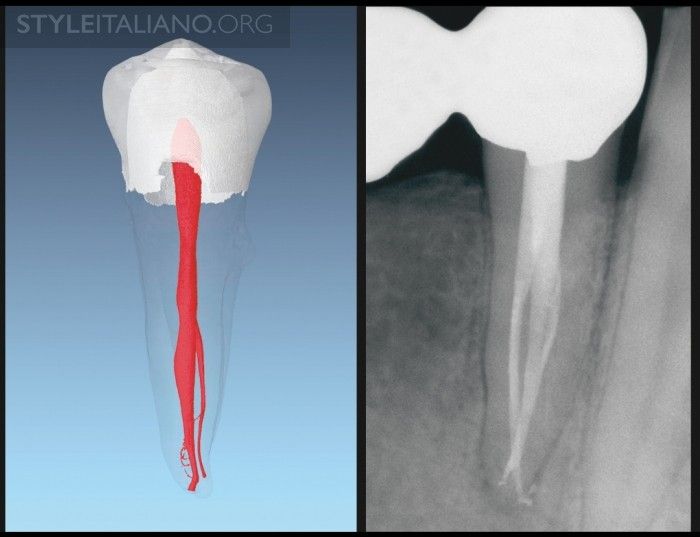
Fig. 2
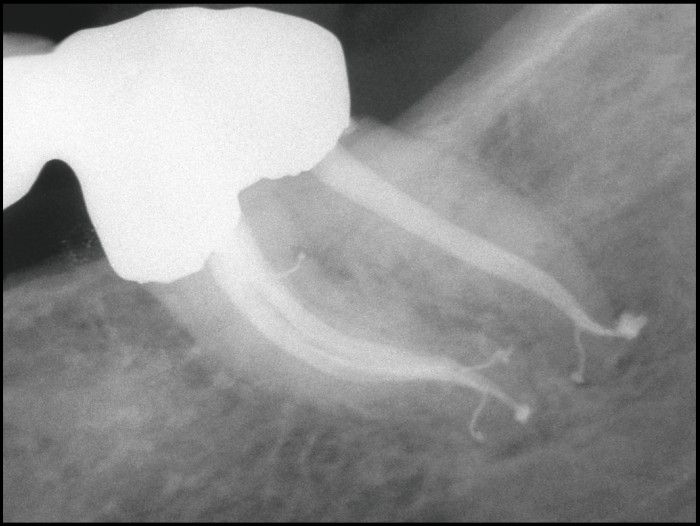
Img. 2 - A µCT image of a mandibular bicuspid (courtesy of Dr. Frank Paque; Zurich, Switzerland). A post-operative film demonstrates similar complex anatomy.
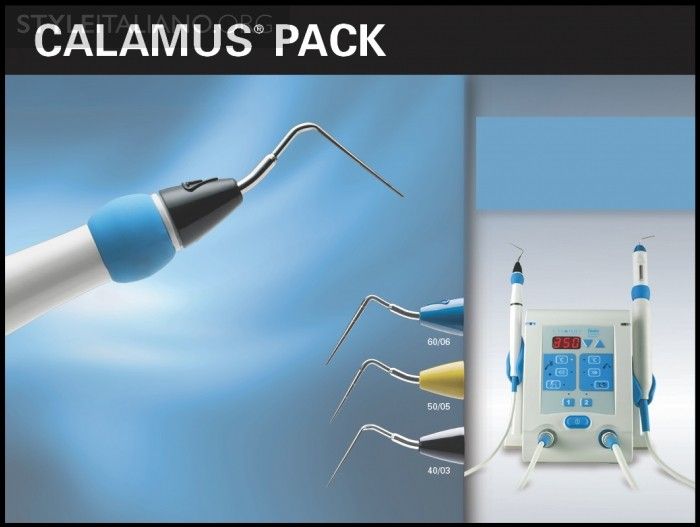
Fig. 3
Img. 3a - The Calamus Pack handpiece (Dentsply Sirona) utilizes a pre-selected Electric Heat Plugger (EHP) to thermosoften and condense gutta percha.
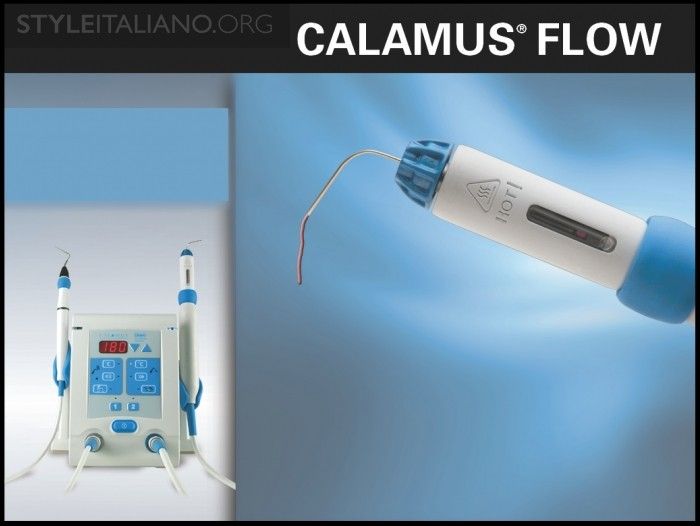
Fig. 4
Img. 3b - The Calamus Flow handpiece (Dentsply Sirona) may be utilized to dispense thermosoftened gutta percha through variably gauged canulas.

Fig. 5
Img. 4 - This image demonstrates a long-standing hand rolling method utilized to fabricate a GPMC.
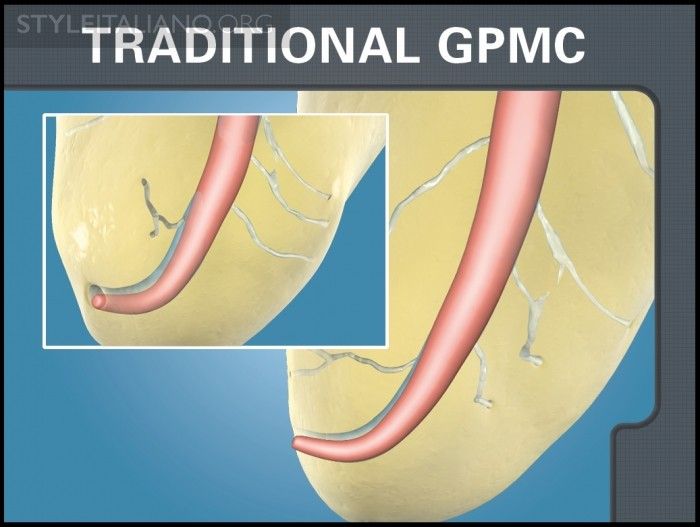
Fig. 6
Img. 5 - Fixed-tapered GPMCs with loose tolerances oftentimes bind in the body of the canal, fit loose at length, and clinically exhibit false apical tugback.
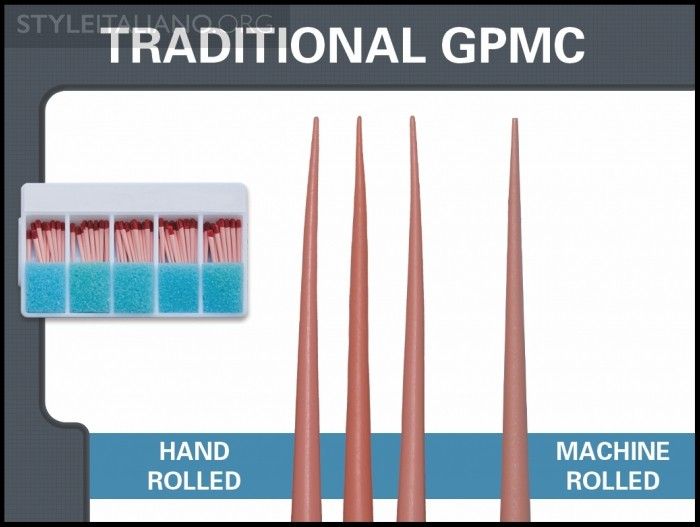
Fig. 7
Img. 6 - Traditional fixed-tapered GPMCs exhibit considerable deficiencies in sizing, clinically contributing to unpredictable cone fit.
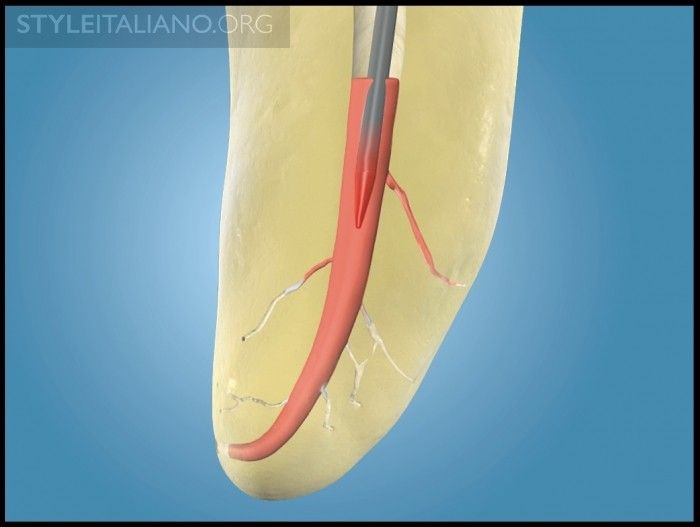
Fig. 8
Img. 7a - Upon activation, an EHP plunges into the body of a GPMC, and sends a thermosoftened heat wave to the apical extent of this cone.
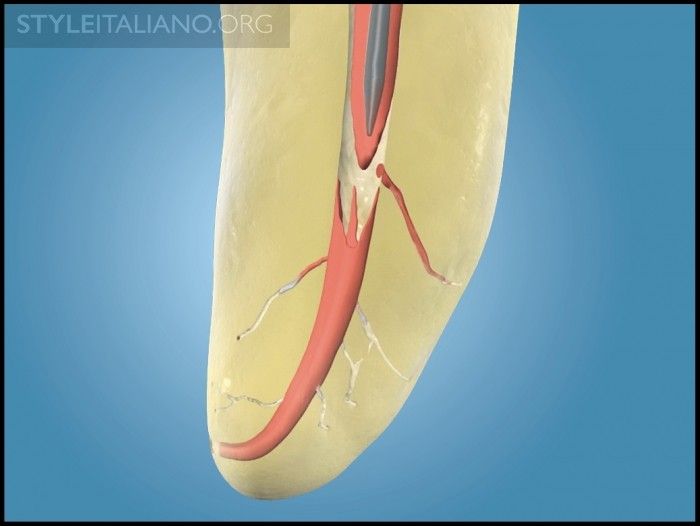
Fig. 9
Img. 7b - Upon deactivation, the cooling EHP is removed, along with a “bite” of gutta percha. This cycle serves to promote an apical wave of condensation.
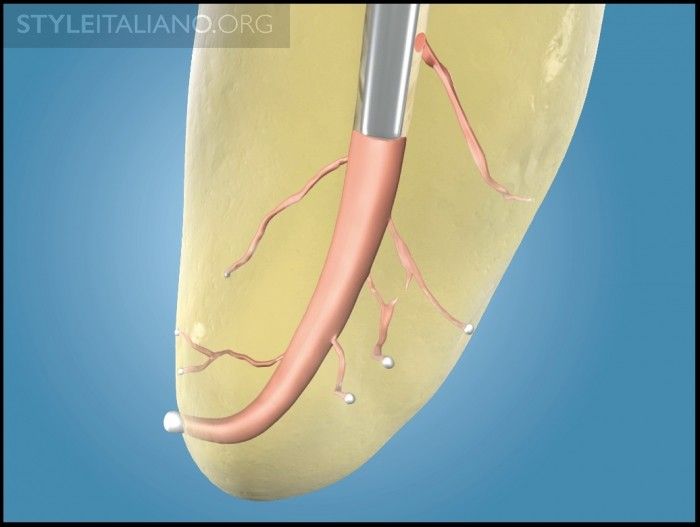
Fig. 10
Img. 7c - A pre-fit plugger compacts warm gutta percha into this region of the root canal system and presses on the cooling mass to offset shrinkage.
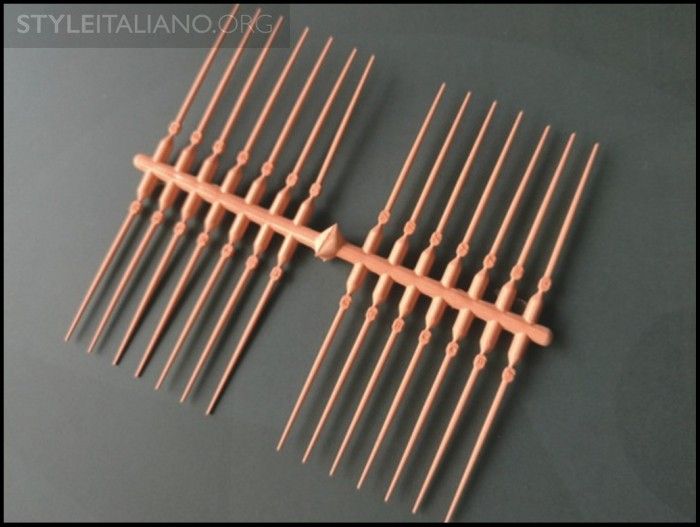
Fig. 11
Img. 8 - Electro-discharge machining is utilized to make precision mold cavities, which, in turn, produce GPMCs with super tight tolerances of ±0.02 mm.
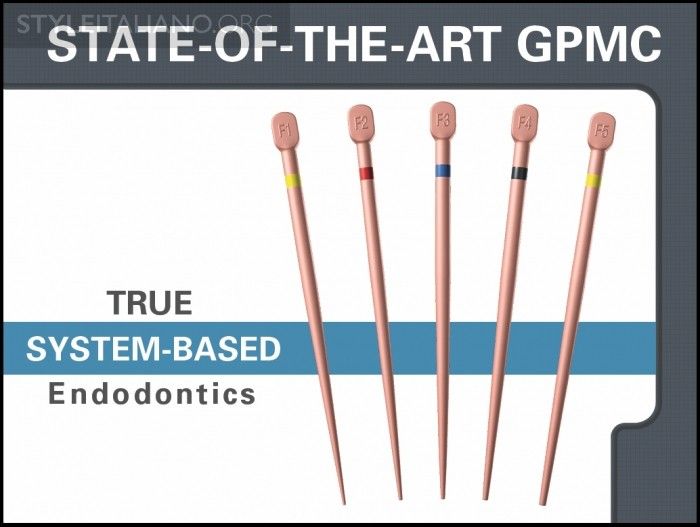
Fig. 12
Img. 9 - The new nano synthetic system-based GPMCs are multi-tapered and superbly sized to match the largest shaping file carried to length.
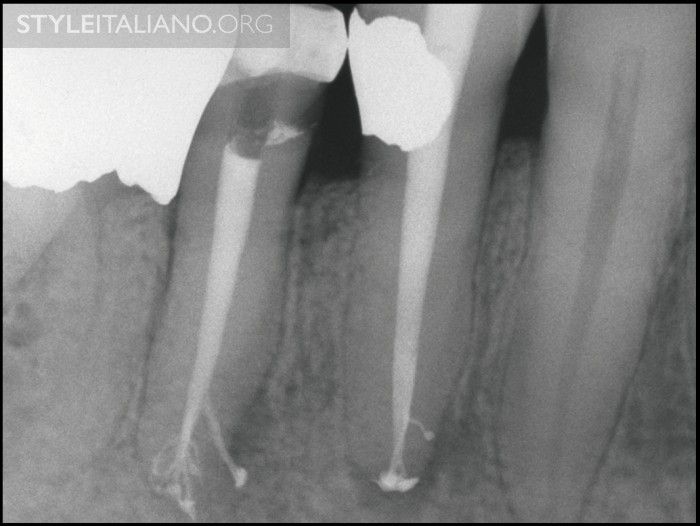
Fig. 13
Img. 10 - A post-treatment image of these mandibular bicuspids demonstrates filling complex root canal systems that exhibit multiple apical portals of exit.
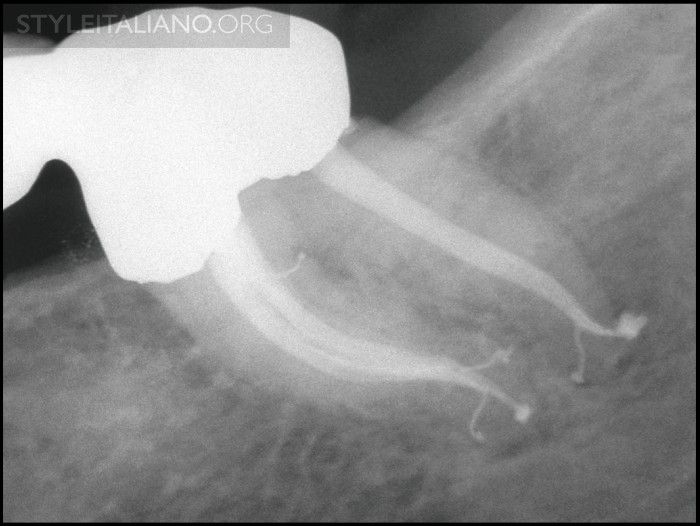
Fig. 14
Img. 11 - This radiograph demonstrates endodontically prepared canals exhibiting multiplanar shapes, and a filled furcal canal and multiple apical portals of exit.
Conclusions
Albert Einstein said, We can't solve problems by using the same kind of thinking we used when we created them.
Now, due to imagination, critical thinking, and recent technological breakthroughs, system-based endodontics is no longer just a concept; rather, system-based endodontics has now become a clinical reality. In the immediate future, there will be an unprecedented endodontic awakening and transformation with the emergence of new technologies that can truly both 3D disinfect and fill root canal systems. Regarding this future, there is an old expression, Whatever you thought, think again.
Bibliography
Grossman LI: Root Canal Therapy Philadelphia: Lea & Febiger, p. 189, 1940.
Schilder H: Filling root canals in three dimensions, Dent Clin North Am pp. 723-744, November 1967.
Marlin J, Schilder H: Physical properties of gutta percha when subjected to heat and vertical condensation, Oral Surg Oral Med Oral Pathol 36:3, pp. 872-879, December 1973.
Goodman A, Schilder H, Aldrich W: The thermomechanical properties of gutta-percha. II. The history and molecular chemistry of gutta-percha, Oral Surg Oral Med Oral Pathol 37:6, pp. 954-961, June 1974.
Goodman A, Schilder H, Aldrich W: The thermomechanical properties of gutta-percha. IV. A thermal profile of the warm gutta-percha packing procedure, Oral Surg Oral Med Oral Pathol 51:5, pp. 544-551, May 1981.
Ruddle, CJ: An in vitro scanning electron microscope study of the warm gutta percha with vertical condensation technique, Thesis, Harvard School of Dental Medicine, 1976.
Ruddle CJ: Ch. 9, Three-dimensional obturation: the rationale and application of warm gutta percha with vertical condensation. In Pathways of the Pulp, 6th ed., Cohen S, Burns RC, eds., St. Louis: Mosby Yearbook Co., 1994.