Irrigants: solutions and techniques
28/05/2016
Paolo Generali
Warning: Undefined variable $post in /var/www/vhosts/styleitaliano-endodontics.org/endodontics.styleitaliano.org/wp-content/plugins/oxygen/component-framework/components/classes/code-block.class.php(133) : eval()'d code on line 2
Warning: Attempt to read property "ID" on null in /var/www/vhosts/styleitaliano-endodontics.org/endodontics.styleitaliano.org/wp-content/plugins/oxygen/component-framework/components/classes/code-block.class.php(133) : eval()'d code on line 2
The aim of root canal therapy is either the treatment or the prevention of periapical periodontitis (or better, periradicular, taking into account lateral lesions). Kakehashi and coworkers, in 1965, were the first to demonstrate the relationship between periradicular lesions of endodontic origin and microbiota in the endodontic space. This result was confirmed later by most cited papers by Bergenholz in 1974 and Sundqvist in 1976. Since periradicular inflammation is usually caused by microbes within the root canal, the goal of endodontic treatment should be the complete disinfection of endodontic space. The disinfection can be achieved only by chemomechanical means, because the complex internal anatomy of the teeth prevents the possibility to reach all the internal walls with instrumentation, either manual, rotary or reciprocating.
Proper cleaning, shaping and filling of the root canal system, as well as the presence of a good coronal seal are key requirements for ensuring the periapical health. Longitudinal studies show that endodontic treatment can achieve high percentage of success, but epidemiological studies conducted in several countries have assessed an high prevalence of periradicular periodontitis in endodontically treated teeth, ranging from 21.5% to 64.5%. One of the key factors is the persistance of bacteria in the endodontic space, or the contamination during the endodontic treatment or later, because of an incomplete seal of the coronal restoration. First of all, such contamination should be prevented, by the use of an aseptic technique, including obviously the rubber dam; this improves the outcome of endodontic treatment. More, failure to use rubber dam can negatively influence the choice of endodontic irrigants.
The topic of endodontic irrigants has been treated extensively in dental literature; more than 1000 papers are listed in PubMed, and a lot of chemicals have been proposed for endodontic disinfection, including extracts of neem leaf, grape seed, aloe vera, garlic, propolis, passion fruit, morinda citrifolia, azadirachta indica and other herbs. However, the gold standard is still Sodum Hypochlorite; other widely used or studied products include chlorexidine, hydrogen peroxide and mixtures of antibiotics, disinfectants and acidic substances.
Unfortunately, most if not all of these products have been studied only in vitro; only 17 papers are available about in vivo studies of irrigants, four of them are randomized clinical trials and one nonrandomized, and there is a lack of agreement between the results. In vitro and ex vivo models should be used only for screening for the best candidates for in vivo screening, and their results should not influence clinical decisions; so, the available evidence on this topic is scarce, and the findings of studies were not consistent. In the hierarchy of evidence, however, in vitro researches are usually ranked well below expert opinions. Surveys have been conducted among general practitioners in different countries, finding out that about 93% used Sodium Hypochlorite as primary irrigant, and the results were similar among Endodontists, showing that the favourite concentration was 5% or higher.
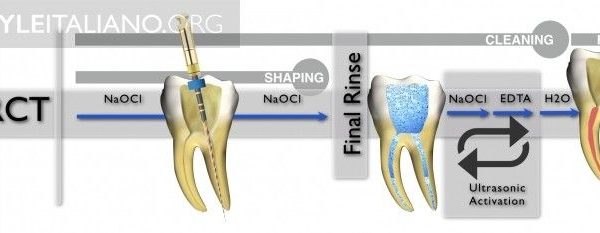
So, it can be stated that there is a widespread agreement that sodium hypochlorite 5% is the most commonly used irrigant, and this makes sense, because of its characteristics: it has a broad spectrum of antimicrobial activity, high organic tissue-dissolving ability, does not leave toxic residues, is inexpensive and fast acting, removes dried or fixed organisms and biofilms from surfaces, and has a low incidence of serious toxicity. Its disadvantages are mostly the danger of accidental extrusion into periapical tissues, accidental ingestion with oropharyngeal burns, accidental ocular irritation, corrosiveness to metals, release of toxic substances when mixed with other chemicals. Sodium hypochlorite presents high surface tension, and this can limit its penetration in the anatomic irregularities of the root canal system and deeper penetration into dentinal tubules, and reduce the contact of the irrigant with the dentin walls and debris. L. Giardino and coworkers have recently proposed to add surfactants to NaOCl, thus enhancing the ability of NaOCl to dissolve organic material. One of the key factors in irrigation is not the efficacy of the solution itself, but the penetration in the apical portions, lateral canals, isthmuses and so on, where it can kill the bacteria and dissolve the tissues. The solution is soon inactivated by various factors, and so it needs to be exchanged frequently. Endodontic instruments must create space, in order to allow efficient penetration of the irrigants; also the time of contact of irritant with dentinal wall and debris is important. Sodium Hypochlorite so must be agitated and exchanged by mechanical, hydrodynamic, sonic/ultrasonic or other methods.
Fig. 1
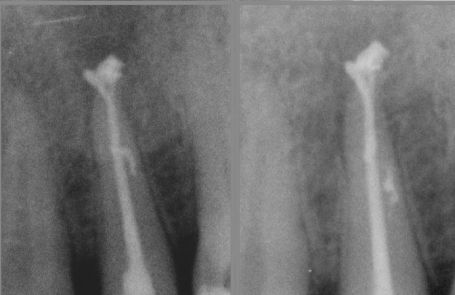
Fig. 2
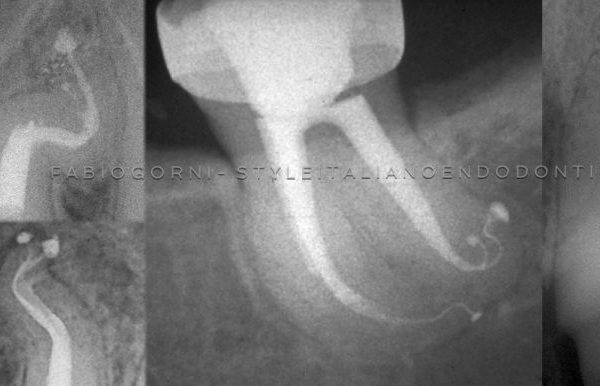
Apical delta and lateral canals are cleaned through the action of irrigants.
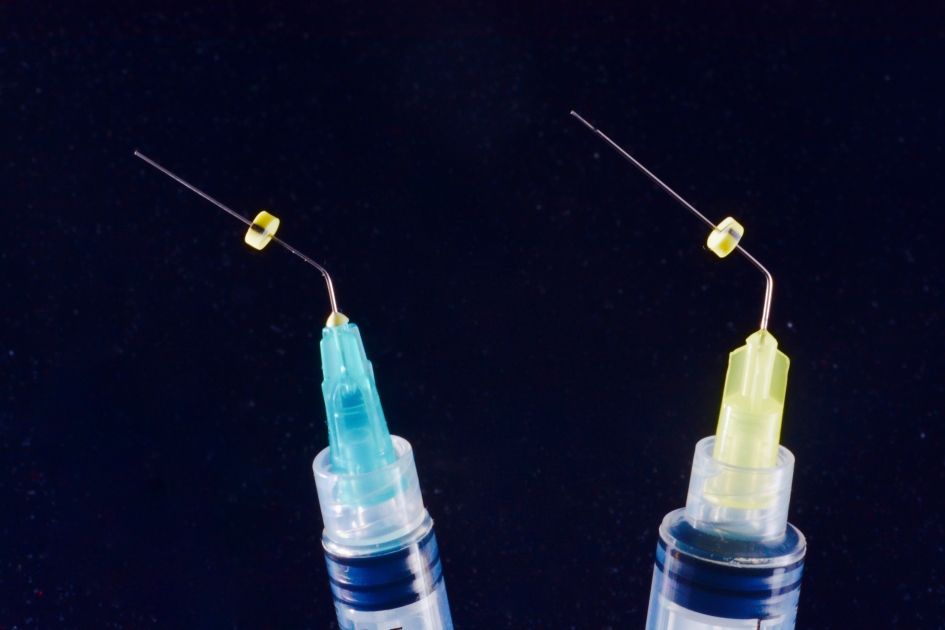
Fig. 3
Needles and syringes for endodontic irrigation have precise characteristics, for efficacy and safety. Open-ended needles are more efficient in irrigant replacement, but create a jet toward the apex, dangerous for extrusion; so they must be kept 3 mm away from apex. Side-vented needles are safer and can be used at 1-2 mm from apex. The needle should be bended at fixed length, e.g. 21, 25 and 28 mm, for easier use, and a silicone stop should be used. Colour-coded needles and syringes are useful for different irrigants. Luer-lock syringe prevents accidental disconnections and spilling.
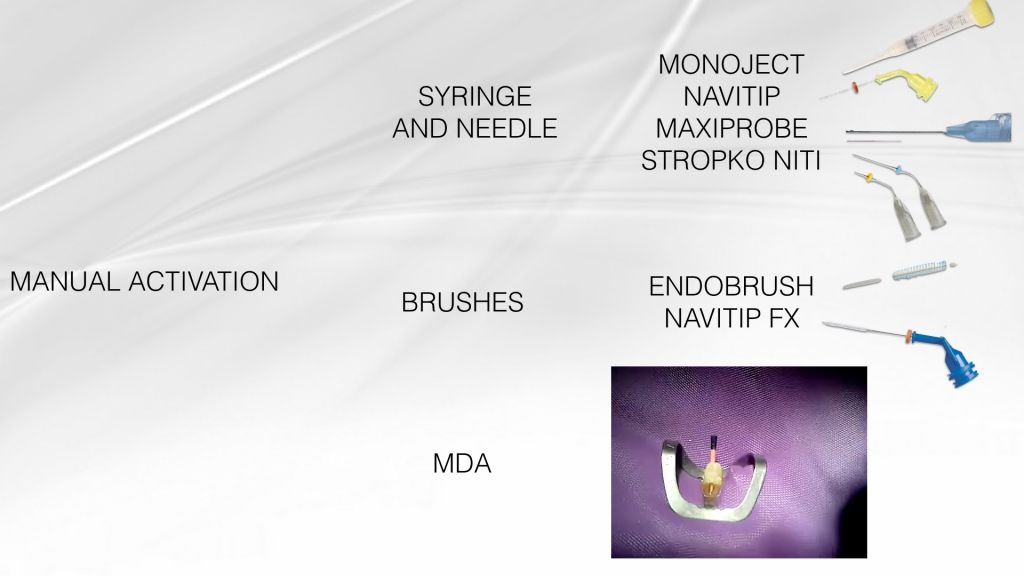
Fig. 4
The efficiency of irrigation can be compromised because of the root canal system complex anatomy, with the lateral canals, isthmuses, ramifications and apical delta. Numerous techniques of active irrigation have been proposed, in order to increase the effectiveness in cleaning the root canal system. Dedicated needles and syringes, brushes or even a guttapercha cone (Manual Dynamic Activation, Caron et al. 2010) can be used manually to improve NaOCl efficacy through mechanical agitation.

Fig. 5
A rotary handpieceattached microbrush has been proven effective in vitro to displace residual debris out of the canal. Continuous irrigation during instrumentation is used by Quantec-E and Self-Adjusting File irrigation system. The EndoVac system is a negative pressure irrigation system, and also RinsEndo uses a pressure-suction system, but with a dedicated disinfectant. The EndoActivator is a sonic device that uses polymer tips to agitate irrigant, while passive ultrasonic irrigation vibrates an endodontic file in the irrigant solution, without simultaneous instrumentation. Irrigants can also be activated through energy delivered by laser.
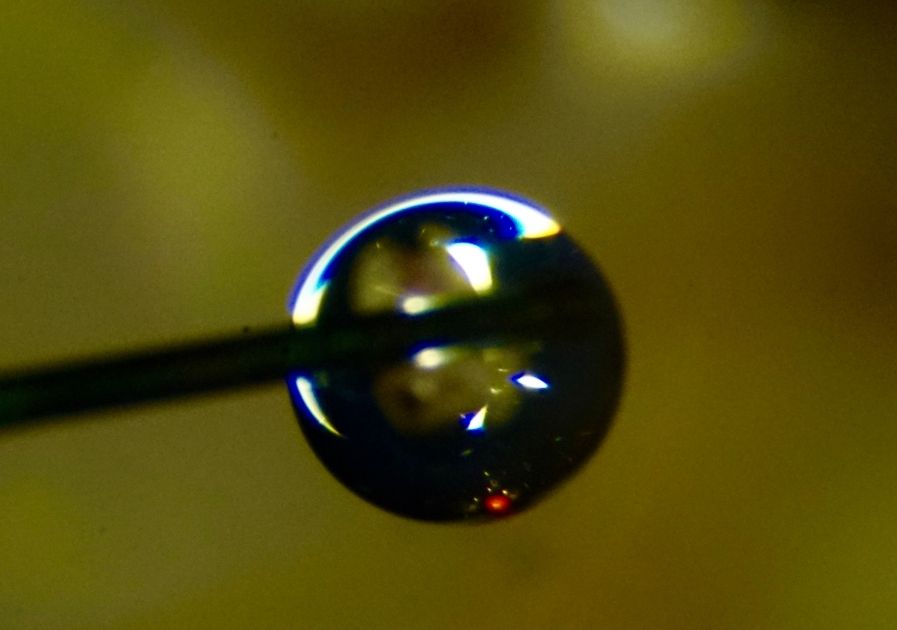
Fig. 6
Microphotography of a drop of irrigant extruded by an endodontic needle.
Conclusions
The ideal properties of an irrigant solution should be: lubrification, suspension of debris, ability to dissolve organic and inorganic tissues, antibacterical and antimicrobial effect, ability to remove bacterial biofilm and to avoid alteration of dentinal structure. NaOCl 5/7%, associated with a chelating agent like EDTA, is the most used irrigant solution and has a strong effect in reducing bacteria inside the canal and as a solvent for organic tissues, while the chelating agent helps in removing smear layer. Heating of Hypochlorite can enhance its effect. A good irrigation technique is of utmost importance, and it starts with a perfect field isolation, the use of a chelating agent during negotiation, an adequate shaping of the root canals to create space for penetration of the irrigant, sufficient volume of irrigant and time, frequent exchange and activation with one of the methods listed above. A final irrigation protocol should be followed after shaping, this is the one used by the author of this article: NaOCL 5% 45° at working length -2mm, activate, suction, repeated three times; suction, saline solution, then EDTA 17%, and final rinse with ethanol.
Bibliography
- Kakehashi S, Stanley HR, Fitzgerald RJ. The effects of surgical exposures of dental pulps in germ-free and conventional laboratory rats. Oral Surg Oral Med Oral Pathol 1965 20(3):340-49.
- Bergenholtz G. Micro-organisms from necrotic pulp of traumatized teeth. Odontol Revy. 1974;25(4):347-58.
- Sjögren U, Figdor D, Persson S, Sundqvist G. Influence of infection at the time of root filling on the outcome of endodontic treatment of teeth with apical periodontitis. Int Endod J. 1997;30:297306.
- Senia ES, Marshall FJ, Rosen S. The solvent action of sodium hypochlorite on pulp tissue of extracted teeth. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1971; 31:96103.
- Generali P, Vellani CP, Tozzi S, Ciacci L, Bertani P. Prevalenza di parodontiti apicali croniche e qualità dei trattamenti endodontici in una popolazione adulta italiana. G It Endo. 2007;21(1):3540.
- McGill S, Gulabivala K, Mordan N, et al. The ef?cacy of dynamic irrigation using a commercially available system (RinsEndo) determined by removal of a collagen
biomolecular ?lm from an ex vivo model. Int Endod J 2008;42:6028. - Ruddle CJ. Endodontic disinfection: tsunami irrigation. Endod Pract 2008;11:715.
- Jiang LM, Verhaagen B, Versluis M, et al. Evaluation of a sonic device designed to activate irrigant in the root canal. J Endod 2010;36:1436.
- Gu LS, Kim JR, Ling J, et al. Review of contemporary irrigant agitation techniques and devices. J Endod 2009;35:791804.
- Yana Y. An in vivo comparative study of the penetration of sodium hypochlorite in root canal systems during cleaning and shaping procedures using the B.U. technique
and sonic instrumentation [masters thesis]. Boston, MA: Boston University; 1989. - Saito K, Webb TD, Imamura GM, et al. Effect of shortened irrigation times with 17% ethylene diamine tetra-acetic acid on smear layer removal after rotary canal instru-
mentation. J Endod 2008;34:10114. - Boutsioukis C, Verhaagen B, Versluis M, Kastrinakis E, Wesselink PR, van der Sluis LWM Evaluation of irrigant flow into the root canal using different needle types by an unsteady computational fluid dynamics model. J Endod 2010; 36: 8759.
- Naenni N, Thoma K, Zehnder M. Soft tissue dissolution capacity of currently used and potential endodontic irrigants. J Endod. 2004;30:785-7.
- Giardino L, Morra M, Becce C, Pappen FG, Mohammadi Z, Palazzi F Comparative wettability of different sodium hypochlorite solutions. G It Endo 2012; 26,5762.