Revascularization of Immature Permanent Teeth With Apical Periodontitis
02/07/2020
Khalid Jamal Attar
Warning: Undefined variable $post in /var/www/vhosts/styleitaliano-endodontics.org/endodontics.styleitaliano.org/wp-content/plugins/oxygen/component-framework/components/classes/code-block.class.php(133) : eval()'d code on line 2
Warning: Attempt to read property "ID" on null in /var/www/vhosts/styleitaliano-endodontics.org/endodontics.styleitaliano.org/wp-content/plugins/oxygen/component-framework/components/classes/code-block.class.php(133) : eval()'d code on line 2
As the Tissue engineering is a growing field ,it will probably be possible to generate a complete vital tooth from a single stem cell . Stem cells are in fact to totipotent cells , which have the capacity to proliferate and to produce cells, which are capable of differentiating into specialized cells. There are two types of stem cells exist: embryonic stem cells and adult stem cells (or postnatal cells). Concerning pulp revascularization, mature stem cells are rather of interest. These cells are found in many sites of the dental element: in the pulp, in the apical papilla, and in the periodontal ligament . In addition, the pulp, which is a product from migration of the neural crest, would probably be a very good candidate to allow nerve regeneration.
The pulp revascularization is dependent on the ability of residual pulp , apical and periodontal stem cells to differentiate. These cells have the ability to generate a highly vascularized and a conjunctive rich living tissue. This one is able to colonize the available pulp space . Subsequently, these stem cells will differentiate into newly formed odontoblasts that will induce an apposition of hard tissue. The nature of this latter is unknown yet.
The pulp revascularization allows also the stimulation of the apical development and the root maturation of immature teeth ( growth and thickening of dentinal walls ).
The presence of deep caries or trauma inducing a stop in the development of root canal of an immature tooth have consider the main indications for the pulp revascularization. It is important to keep in mind that an endodontic treatment of immature tooth, often necessary up to now , involves root canal treatment on an open apex tooth with thin and fragile walls. This will involve the persistence of a weakened tooth that may not provide a long-term prognosis due to there significance of an intrinsic fragility and the difficulty to obtain a good sealing at the open apex. Revascularization technique would allow the growth of root with dentinal walls thicken which reduce the risk of root fracture.
A 10- year-old male visited our dental office suffering from sever pain after 2 weeks from previous endodontic treatment (teeth #11 and #12 ) .
Upon clinical examination at our clinic, temporary fillings were found in the palatal surfaces of teeth #11 and #12, without any tooth discoloration and no evidence of sinus tract fistula .These 2 teeth were sensitive to percussion and palpation and did not respond to thermal or electric pulp testing (Vitality Scanner; Sybron-Endo, Glendora, CA).
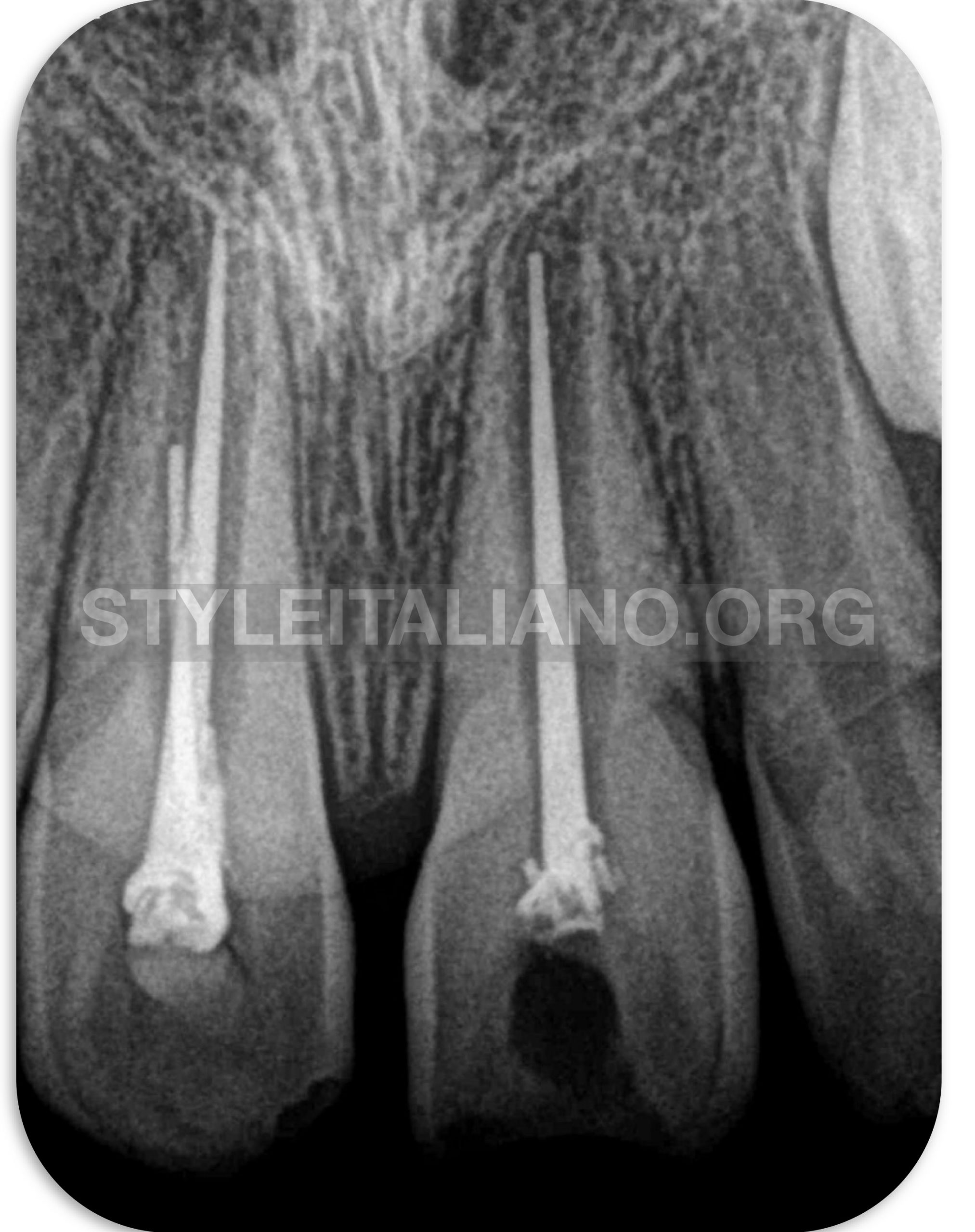
Fig. 1
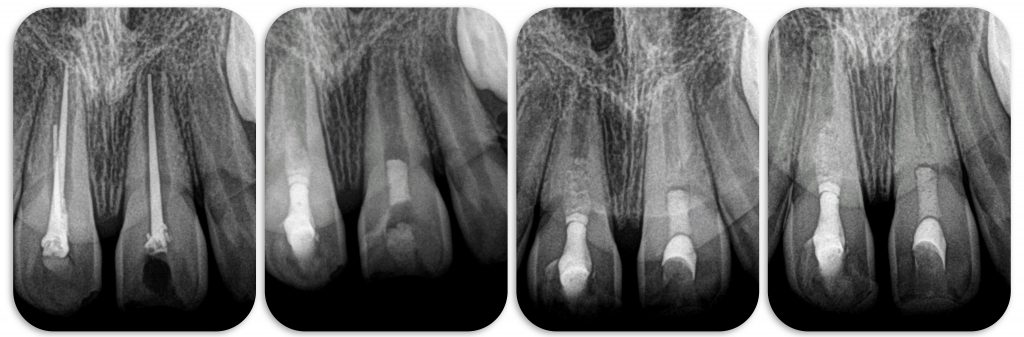
Periapical radiographic examination revealed bad previous root canal treatment and incomplete apices on both left and right first maxillary central incisors.
The patient was informed that the goal of treatment was to initiate healing of the peri-apical inflammation and to stimulate further root lengthening and thickening and the proposed treatment might not be successful. The decision was made to perform an endodontic revascularization procedure .Written informed consent was obtained from the patient.
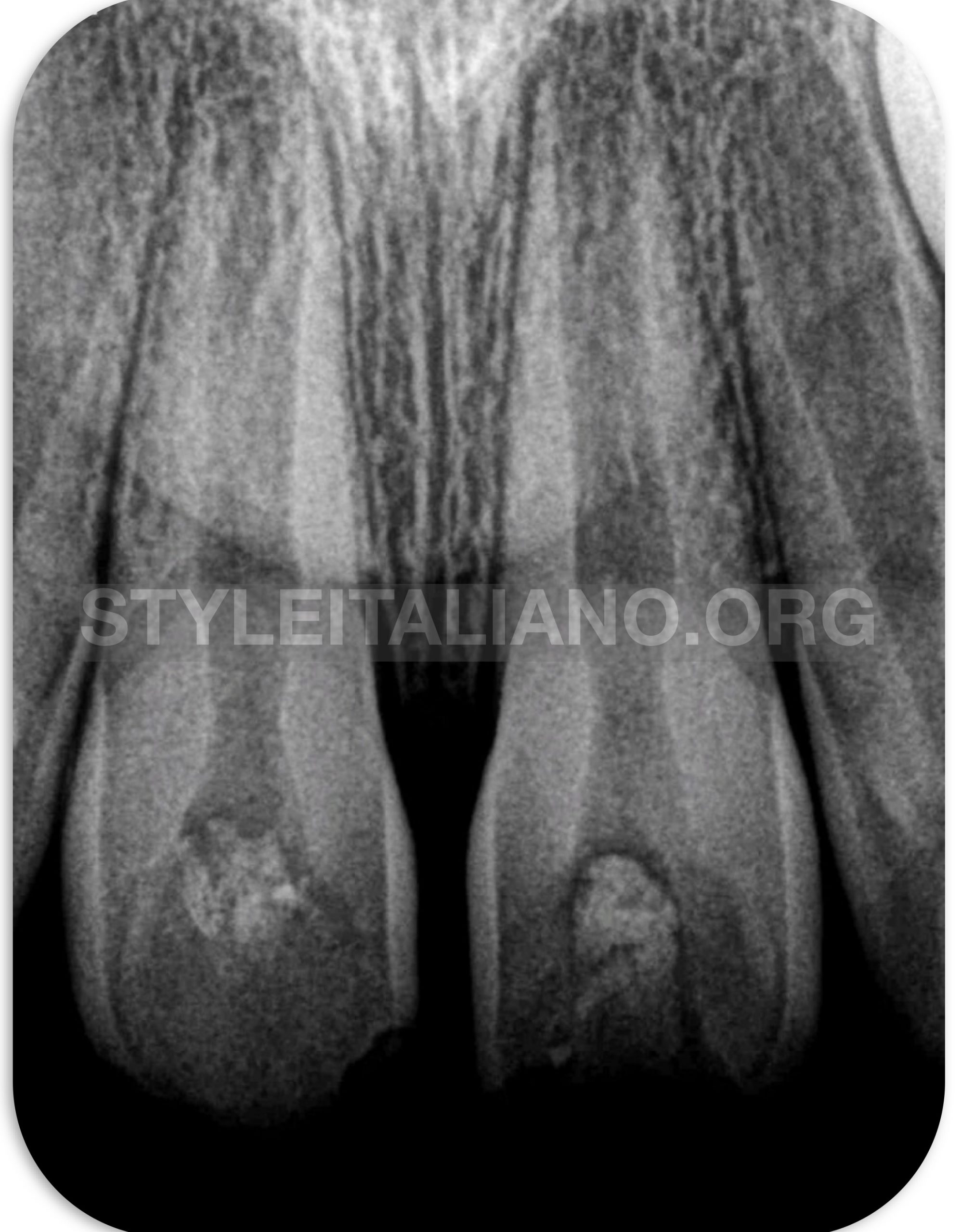
Fig. 2
Under local anesthesia, the tooth was isolated with a rubber dam and the temporary filling was removed. Previous root canal filling material (gutta percha ) was removed by micro-tweezer from Zumax (Zumax Medical Co.Ltd) under microscopic magnification. A 15 K-file was introduced into the canal to establish a working length with periapical radiograph. The remnant necrotic tissue was removed from the root canal by irrigating the root canal gently 17%EDTA followed by 10 mL 2.5% NaOCl warming at37∘C dispensed through a syringe with 2-sided vented irrigation tips IrriFlex ( Produits Dentaires ,Swistzerland ) for 2 min without any further instrumentation .The irrigation tips was introduced into the root canal to a point 2 mm shorter than open apex and NaOCl was slowly expressed from the syringe to prevent its introduction into the periapical tissues. The canal was carefully dried with large, sterile paper points. The root canal was then medicated with triple antibiotic dressing paste. The medicament was made by mixing equal doses of 3 antibiotics (250 mg each): metronidazole, ciprofloxacin and minocycline , with sterile saline to a creamy paste. The dressing was applied using a lentulo-spiral and tapped down into the canal with the blunt ends of sterile paper points to a depth 2 mm short of the root apex without overflow at the pulp chamber to avoid any future staining. The tooth was temporarily restored by placing a sterile cotton pellet over the root canal medicament and then covering the pellet with Cavit cement (3M ESPE, St. Paul, MN,USA). The Cavit was in turn covered with glass ionomer cement that affords the seal greater resistance to occlusal forces and wear during the long intervals that can occur between appointments. The patient was instructed to come for a follow-up visit 2 weeks later.
After 2 weeks, the tooth was free of symptoms.Under rubber dam isolation and local anesthesia without a vasoconstrictor in order not to inhibit the future apical bleeding , the Cavit was removed. The mixture of antibiotics was completely removed with 2.5% NaOCl and sterile saline. The apical tissue was stimulated with 15 K-file to induce bleeding into the pulp canal. Bleeding control was performed at a level 3mm below the cemento-enamel junction. A blood clot formed in the canal about 15 min after simulation. MTA ( Produits Dentaires,Switzerland ) was applied over the blood clot using MAP one system ( ), followed by a moist cotton pellet. Cavit was placed temporarily over the cotton pellet
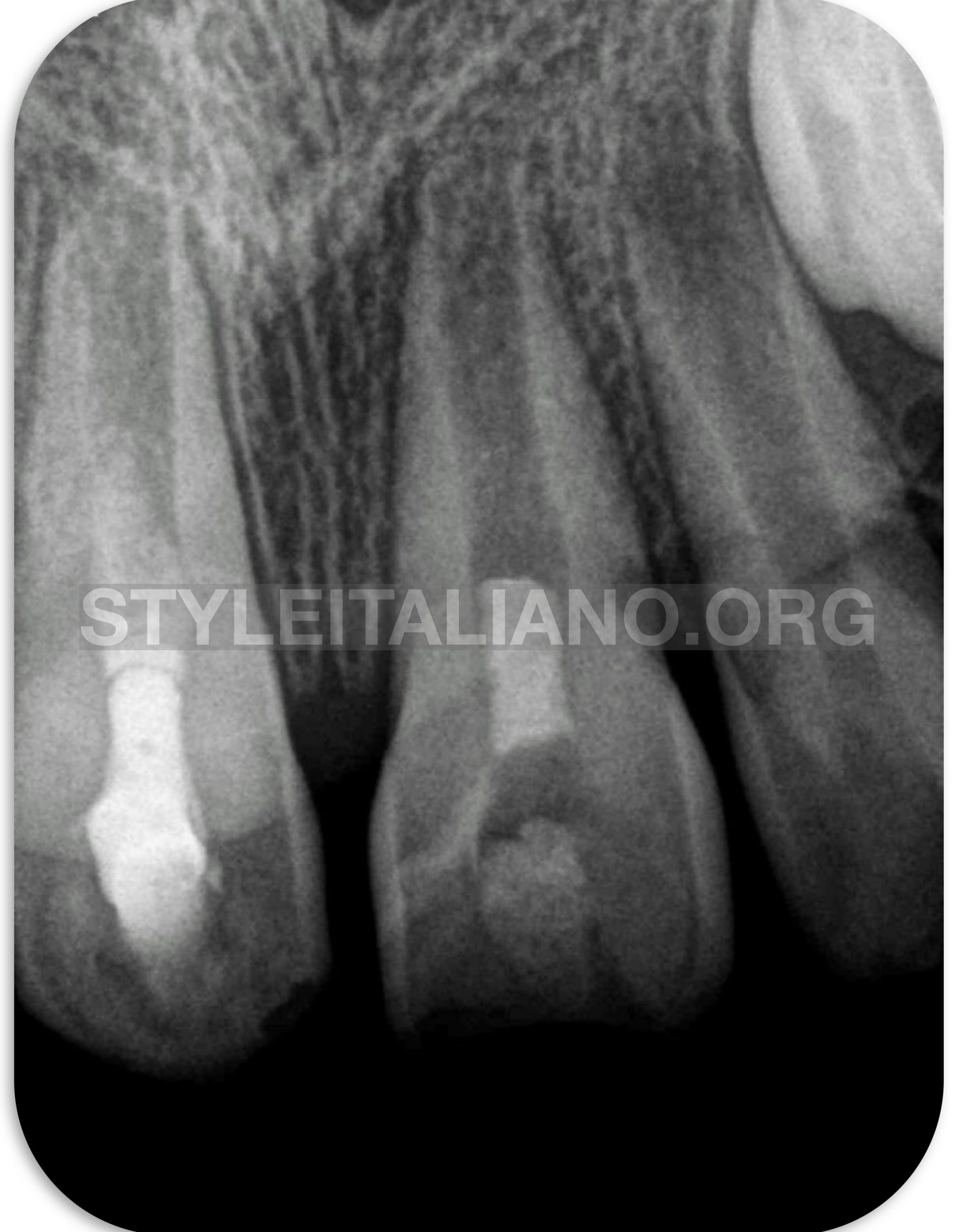
Fig. 3
.One week later, the Cavit and cotton pellets were removed and the coronal accesses were finally sealed with composite resin (Z250; 3M ESPE, St. Paul, MN, USA) figure (3).
The patient was instructed to visit at 2 weeks, then at 6 and 12 months for postoperative follow-up.
Fig. 4
Following the analysis of pulp revascularization approaches , and before opening the tooth , it seems effective to isolate the tooth with a rubber dam and disinfect it with 10% povidone iodine ( iso-Betadine) to ensure the maximum reduction of oral bacterial concentration. The access to the pulp chamber was achieved. No root canal instrumentation is still recommended to avoid altering dentinal walls and stem cells present on their surfaces. However the best way to remove infected and necrotic pulp tissue irrigation with 17%EDTA combined with 6%sodium hypochlorite seems to be the best solution as 17% EDTA (little cytotoxic and opening dentin tubules) allows better penetration of the irrigants (and medications) in root canal crevices and tubules . A release of growth factors imprisoned during dentinogenesis could be expected. Sodium hypochlorite remains irrigator base for root canal disinfection. If we use a 2.5% sodium hypochlorite concentration, its effectiveness and its solvent power may be potentiated by warming at 37∘C. It also seems that rinsing with saline could only have a benefit to treatment. Regarding root canal temporary medication , triple antibiotic paste used has good concentration that seems to be the most appropriate in order to avoid any problems associated with calcium di-hydroxide (weakening dentinal walls, inducing tissue necrosis, and decreasing effectiveness by infectious exudates). Indeed, the three antibiotics has been reported to be successful regimen in controlling the root canal pathogens and show minimum stem cells cytotoxicity when used in adequate concentration. In vitro studies have demonstrated that calcium di-hydroxide and MTA, with their high pH, exert a severe weakening effect on dentin walls during a period of two weeks to two months . However, samples sealed with MTA seem to recover their mechanical properties as fracture toughness after one year.
Conclusions
Recently , clinical and radiographic evidence demonstrated the successful revascularization treatment of immature necrotic permanent teeth with apical periodontitis. This treatment approach offers great potential to avoid the need for traditional apexification with calcium hydroxide or the need to achieve an artificial apical barrier with mineral trioxide aggregate. Furthermore, revascularization allows the stimulation of the apical development and the root maturation of immature teeth (root growth and thickening of dentinal walls and natural apexification).
Bibliography
W.ZhangandP.C.Yelick,“Vitalpulptherapy-currentprogress of dental pulp regeneration and revascularization,” InternationalJournalofDentistry,vol.2010,ArticleID856087,9pages, 2010.
R. Vijayaraghavan, V. M. Mathian, A. M. Sundaram, R. Karunakaran, and S. Vinodh, “Triple antibiotic paste in root canal therapy,”Journal of PharmacyAndBioalliedSciences,vol. 4,supplement2,pp.230–233,2012.
A. Thomson and B. Kahler, “Regenerative endodontics— biologically-based treatment for immature permanent teeth: a case report and review of the literature,”Australian Dental Journal,vol.55,no.4,pp.446–452,2010
K.Reynolds,J.D.Johnson,andN.Cohenca,“Pulprevascularization of necrotic bilateral bicuspids using a modified novel technique to eliminate potential coronal discolouration:acase report,”InternationalEndodonticJournal,vol.42,no.1,pp.84– 92,2009.
M.Torabinejad,R.Corr , M.Buhrley , K.Wright, and S.Shabahang, “An animal model to study regenerative endodontics,” JournalofEndodontics,vol.37,no.2,pp.197–202,2011.
A. Nosrat, A. Seifi, and S. Asgary, “Regenerative endodontic treatment(revascularization)fornecroticimmaturepermanent molars:areviewandreportoftwocaseswithanewbiomaterial,” JournalofEndodontics,vol.37,no.4,pp.562–567,2011
E.G.Trevino,A.N.Patwardhan,M.A.Henryetal.,“Effectof irrigantsonthesurvivalofhumanstemcellsoftheapicalpapilla inaplatelet-richplasmascaffoldinhumanroottips,”Journalof Endodontics,vol.37,no.8,pp.1109–1115,2011.
W. T. Cunningham and S. W. Joseph, “Effect to temperature on the bactericidal action of sodium hypochlorite endodontic irrigant,” Oral Surgery Oral Medicine and Oral Pathology, vol. 50,no.6,pp.569–571,1980.
A. Adl, N. S. Shojaee, and M. Motamedifar, “A comparison between the antimicrobial effects of triple antibiotic paste and calcium hydroxide against entrococcus faecalis,” Iranian EndodonticJournal,vol.7,no.3,pp.149–155,2012.
S. Hatibovi´c-Kofman, L. Raimundo, L. Zheng, L. Chong, M. Friedman, and J. O. Andreasen, “Fracture resistance and histological findings of immature teeth treated with mineral trioxideaggregate,”DentalTraumatology,vol.24,no.3,pp.272– 276,2008
A. P. Leiendecker, Y.-P. Qi, A. N. Sawyer et al., “Effects of calciumsilicate-basedmaterialsoncollagenmatrixintegrityof mineralized dentin,” Journal of Endodontics, vol. 38, no. 6, pp. 829–833,2012.



